Phalloplasty
Neophalloplasty is one of the most difficult surgical procedures in genital reconstructive surgery. It is indicated in men when the penis is missing due to either congenital or acquired reasons, as well as in transmen. Many different tissues have been applied such as local vascularized flaps or microvascular free transfer grafts. The main goal of the neophalloplasty is to construct the functional and cosmetically acceptable penis. Urethral reconstruction in neophalloplasty presents a great challenge for surgeons who manage genital reconstruction. Different flaps (penile skin, scrotal skin, abdominal skin, labial skin, vaginal flaps, etc.) or grafts (skin, bladder, buccal mucosa) have been suggested for urethral lengthening. Although serious complications were reported in the past, new techniques and modifications for primary and secondary neophallus urethroplasty seem to be safe in experienced hands.
Several surgical techniques for neophallic reconstruction have been reported using either available local vascularized tissue or microvascular tissue transfer. However, none of them satisfy all the goals of modern penile construction, i.e. reproducibility, tactile and erogenous sensation, a competent neourethra with a meatus at the top of the neophallus, large size that enables safe insertion of penile implants, satisfactory cosmetic appearance with hairless and normally colored skin. Normal penis has some unique characteristics and restoring its psychosexual function in both the flaccid and erectile state, and the possibility of sexual intercourse with full erogenous sensations, is almost impossible. Surgical indications are expanded to many other disorders such as penile agenesis, micropenis, disorder of sexual development (intersex conditions), failed epispadias or hypospadias repair, penile cancer, as well as female transsexuals.
The most widely used flap for total neophalloplasty is the radial forearm flap. However, it has many drawbacks, e.g. an unsightly donor site scar, very frequent urethral complications, and small sized penis that does not allow the safe insertion of penile prosthesis in majority of cases. This was the main reason for us to develop a new technique using the musculocutaneous latissimus dorsi (MLD) free transfer flap, which mostly satisfies the requirements noted above. Due to its workable size, ease to identification, long neurovascular pedicle and minimal functional loss after removal, the latissimus dorsi flap has been used for a variety of reconstructions. It has a reliable and suitable anatomy to meet the esthetic and functional needs for phallic reconstruction. It can also be used successfully in children. Phallic retraction with muscle based grafts seems less likely to occur than with use of fasciocutaneous forearm flap, since denervated well-vascularized muscle is less prone than connective tissue to contract.
Operative technique
We perform total phalloplasty in transmen in two or three stages and it includes several procedures:
FIRST STAGE (6-7 hours)
1. Removal of internal (uterus, Fallopian tubes, ovaries – transvaginal, laparoscopic or abdominal approach) and external female genitalia (vagina, vulva).
2. Lengthening of the urethra using all available vascularized hairless genital skin.
3. Scrotoplasty with insertion of testicular implants.
4. Musculocutaneous latissimus dorsi / abdominal phalloplasty.
SECOND STAGE (6 months later, 2 – 3 hours)
1. Reconstruction of the neophallic urethra using skin/buccal mucosa graft.
2. Glans reconstruction.
3. Implantation of the semirigid or inflatible penile prosthesis.
THIRD STAGE (if necessary, 2 hours)
1. Urethral tubularisation.
2. Additional correction of all esthetic deformities.
MUSCULOCUTANEOUS LATISSIMUS DORSI (MLD) PHALLOPLASTY
A latissimus dorsi musculocutaneous flap of the non-dominant side is designed and harvested with thoracodorsal artery, vein and nerve. The surface of the flap is templated in two parts: (1) a rectangular part for neophallic shaft to be approximately 15-17 x 12-14 cm and (2) additional, circular or semilunar component for glans reconstruction. The flap is tubularized in the midline and the neoglans formed by folding over and approximating to the penile shaft. The new constructed phallus is detached from the axilla after clamping dividing neurovascular pedicle with aim to achieve maximal pedicle length. The donor site defect is closed by direct skin approximation. If it is impossible, remaining donor site defect is grafted with split-thickness skin graft. Incision is made in the pubic area and a wide tunnel toward the femoral region is created to place the flap pedicle. The neophallus is transferred to the recipient area and microsurgical anastomoses are created between thoracodorsal and femoral artery, thoracodorsal and saphenous vein and thoracodorsal and ilioinguinal nerve. Specially constructed dressing is used to keep the neophallus in an elevated position for approximately two weeks.
Second stage includes implantation of penile prosthesis, either maleable or inflatible, further urethral lengthening and glans reconstruction. Glans is reconstructed using Norfolk technique.
ABDOMINAL PHALLOPLASTY
In this type of phalloplasty, a pedicled fasciocutaneous flap of appropriate dimensions is harvested from the suprapubic region, and tubularised to create a neophallus. Donor area is closed by direct approximation, or using skin grafts. Since this is a pedicled flap, the survival is more reliable then with free flaps. Another advantage is preserved tactile sensitivity of entire neophallus. Disadvantages are limited size of the neophallus, hair growth and it can be performed in selected cases.
Urethral reconstruction
Urethral reconstruction presents the main problem in this type of sex reassignment surgery and includes creation of a very long neourethra, since the native urethral meatus in females is positioned too far from the tip of the glans. Lengthening of the native urethra presents a great challenge, especially the first part that should be the bridge between native meatus and neophallic urethra. Neophallic urethral reconstruction is followed in the second stage and includes complete urethral lengthening (if possible) or placement of the buccal mucosa graft on the ventral side of the neophallus, and later tubularisation (third stage).
Reconstruction of the neourethra starts with reconstruction of its bulbar part. A vaginal flap is harvested from the anterior vaginal wall with the base close to the female urethral meatus. This flap is joined with the remaining part of the divided urethral plate forming the bulbar part of the neourethra. Additional urethral lengthening is performed using all available vascularized hairless tissue to lengthen the neourethra, maximally preventing the postoperative complications. For this reason, both labia minora and available clitoral skin are used for urethral tubularization. This way, the new urethral opening is placed in first half of neophallus, minimizing the requests for longer neophallus urethroplasty. It is always done in the first stage of total phalloplasty.
The most promising technique for the further lengthening of the neophallus urethra is based on two-staged procedure. The first stage includes creation of the new “urethral plate” using buccal mucosa graft. The use of buccal mucosa graft that was first described seven decades ago, has been the gold standard for urethral reconstruction. It is tough, elastic, simple to harvest, easy to handle and leaves no noticeable scar at the donor site. Buccal mucosa grafts (either pairs or single, depending on the width and length of neourethra needed) are placed on the ventral side of the penis. When the healed grafts are ready for final stage tubularization and closure, it is important to incise the underlying tissue that will support the neourethra and avoid ischemia at the neourethral suture line. It is recommended to create second layer from surrounding tissue to cover and support the new created urethra. The key for successful repair is waiting long enough until the skin is supple. The classic mistake is to perform second stage too early.
Second stage should be performed when the “urethral plate” has matured enough to be supple and thus more easily mobilized for a tubularization. If it is necessary, additional buccal mucosa grafts can be used for urethral plate augmentation and easier tubularization.
Complications and secondary repair
Most of the urethral problems can be corrected with secondary procedures. Our experience so far has showed that more than half of urethral fistulas and strictures are solved conservatively, while less than half complications need an additional surgical procedure. At least, there is still no ideal technique for phalloplasty resulting in excellent aesthetic and functional outcomes. There are still problems with the neourethral reconstruction, but the incidence of complications has been reduced with new refinements of one stage repair or by using a staged procedure
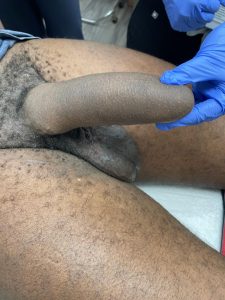
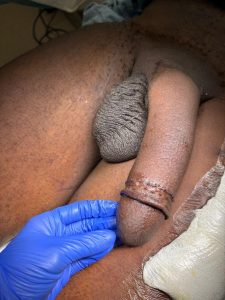
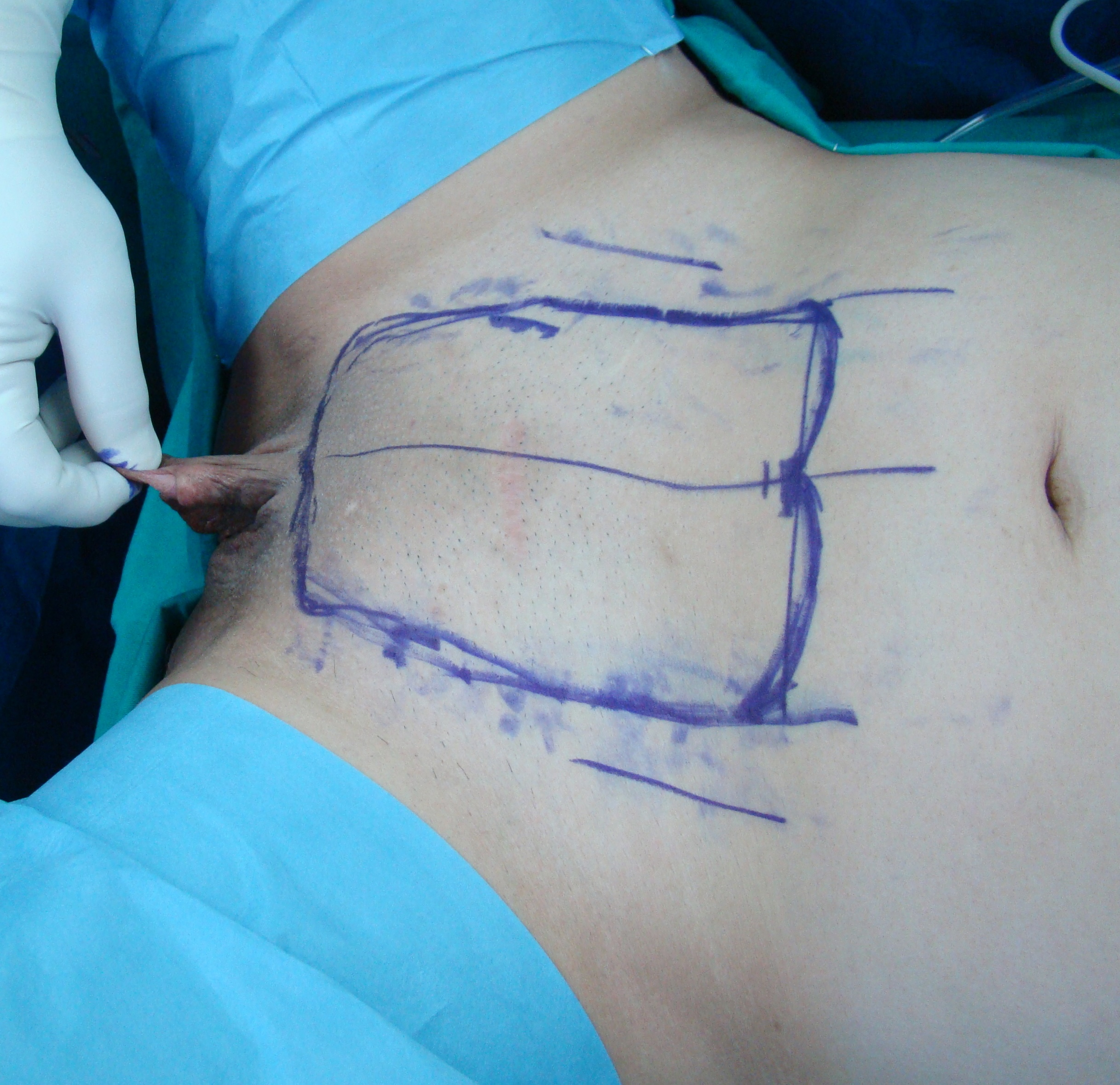
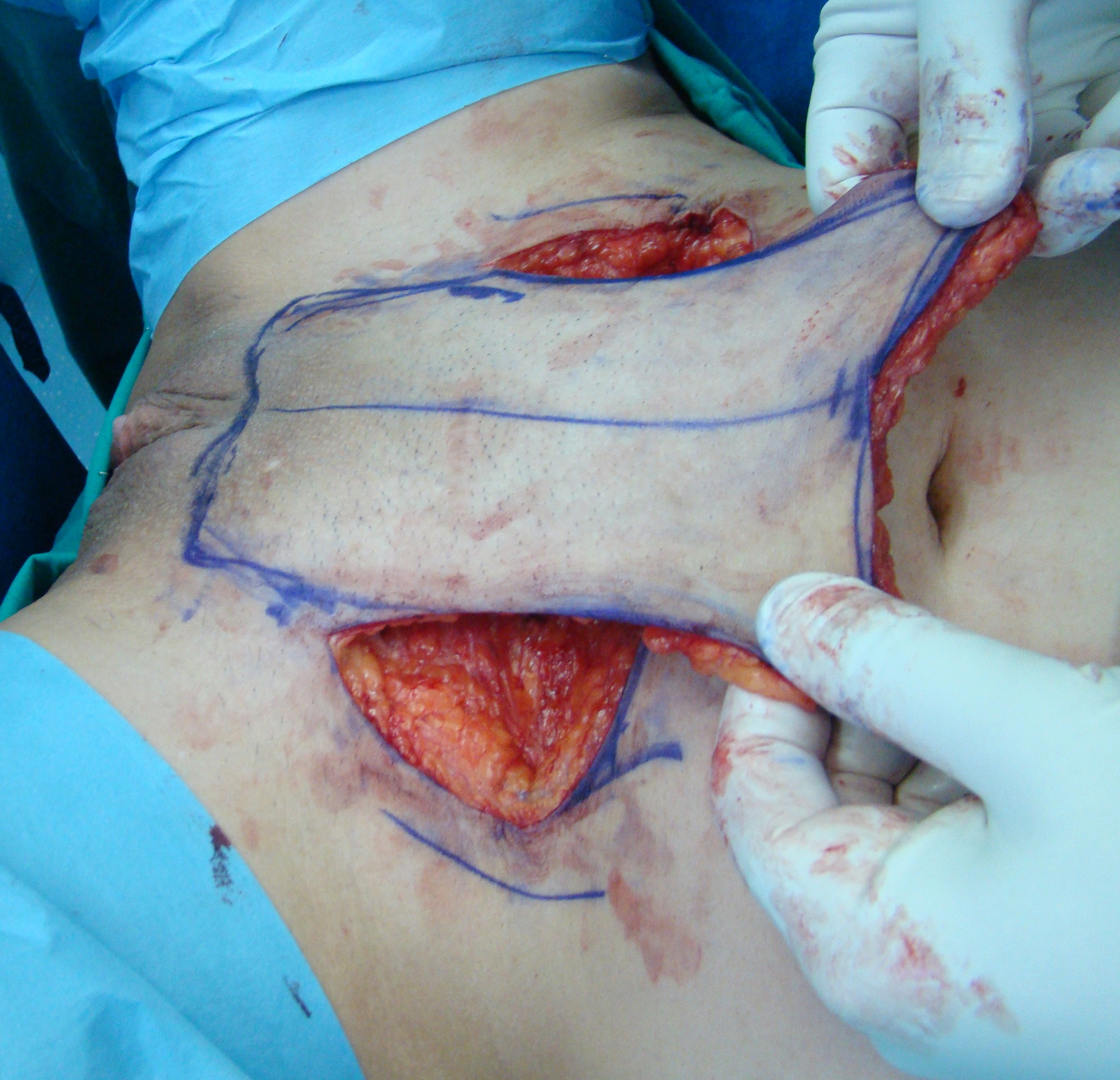
CASE 1
Total MLD (musculocutaneous latissimus dorsi) phalloplasty in transman
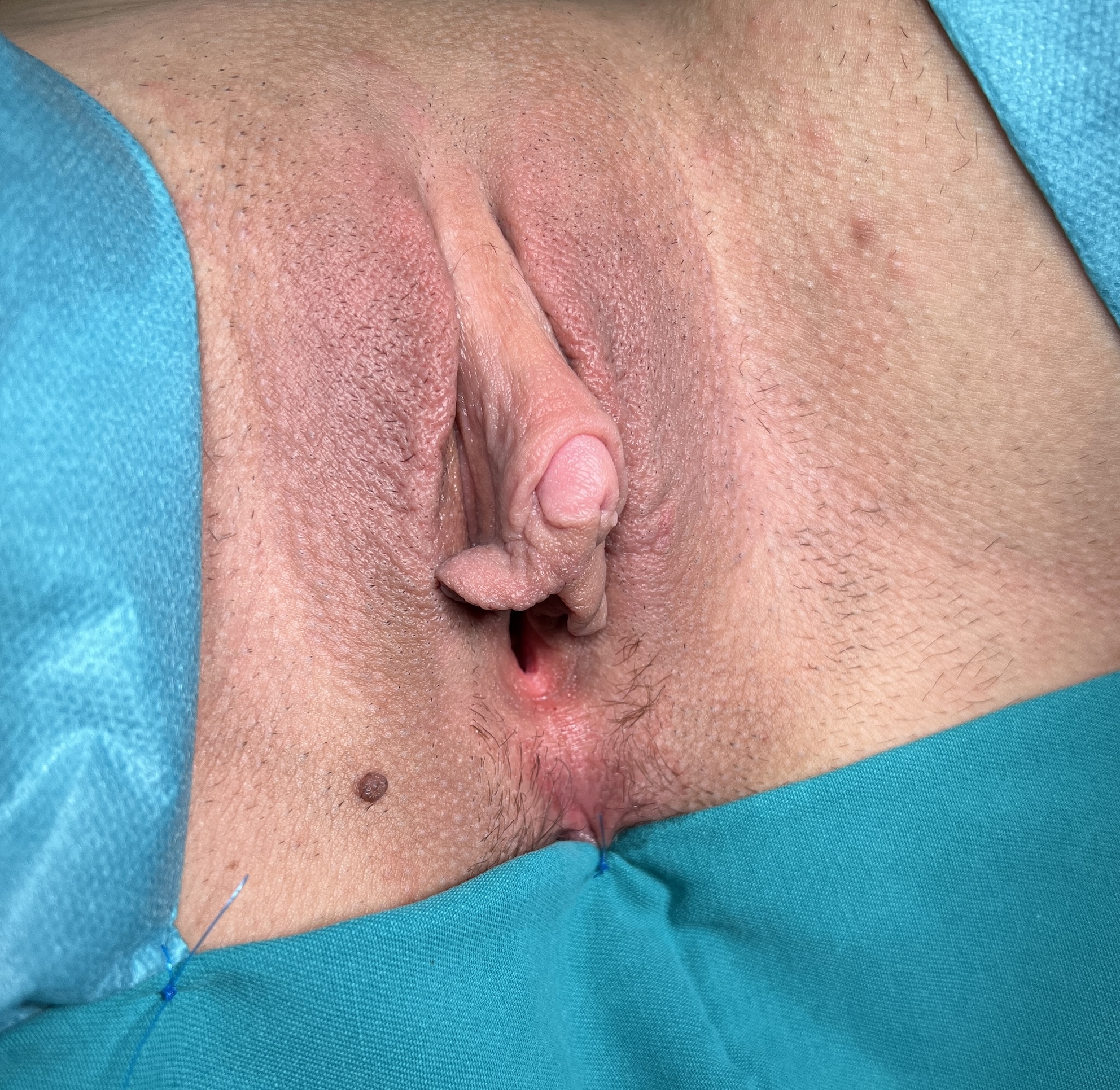 Preoperative appearance.
Preoperative appearance.
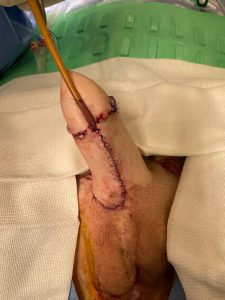
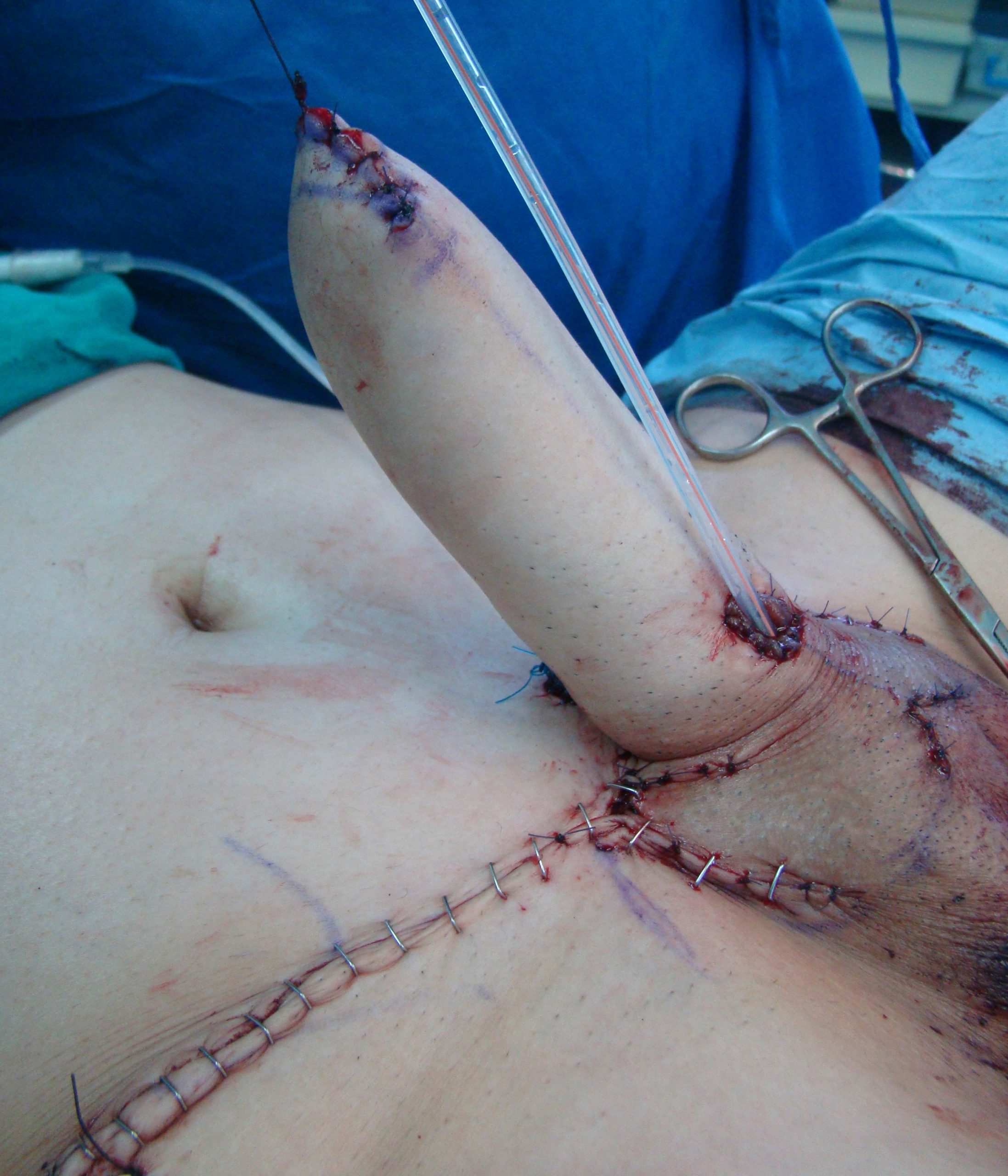
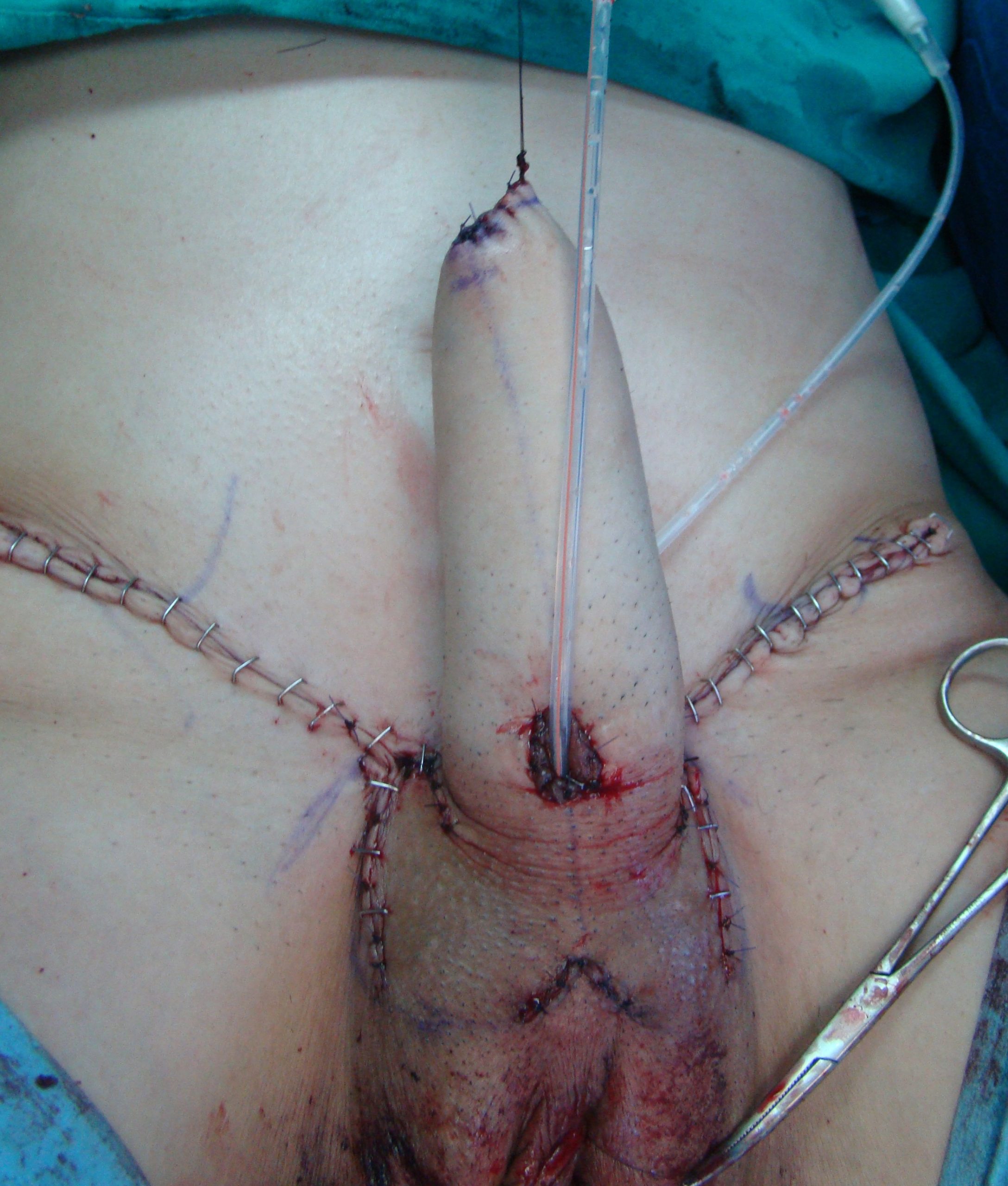
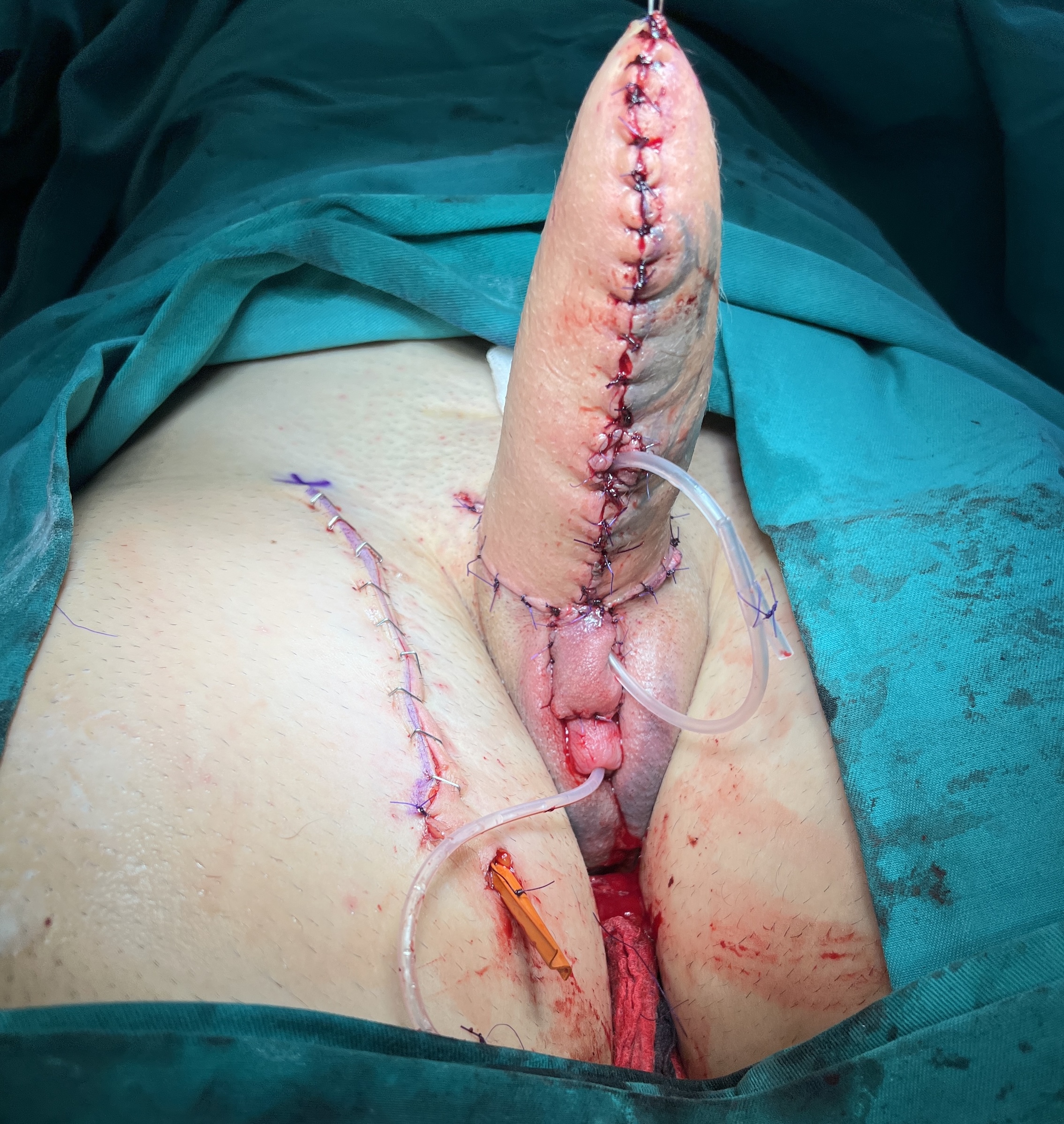 Outcome after surgery. Scrotum with two testicle prostheses is formed. Neophallus is fixed just above the scrotum. Clitoral glans is placed under the base of the neophallus
Outcome after surgery. Scrotum with two testicle prostheses is formed. Neophallus is fixed just above the scrotum. Clitoral glans is placed under the base of the neophallus
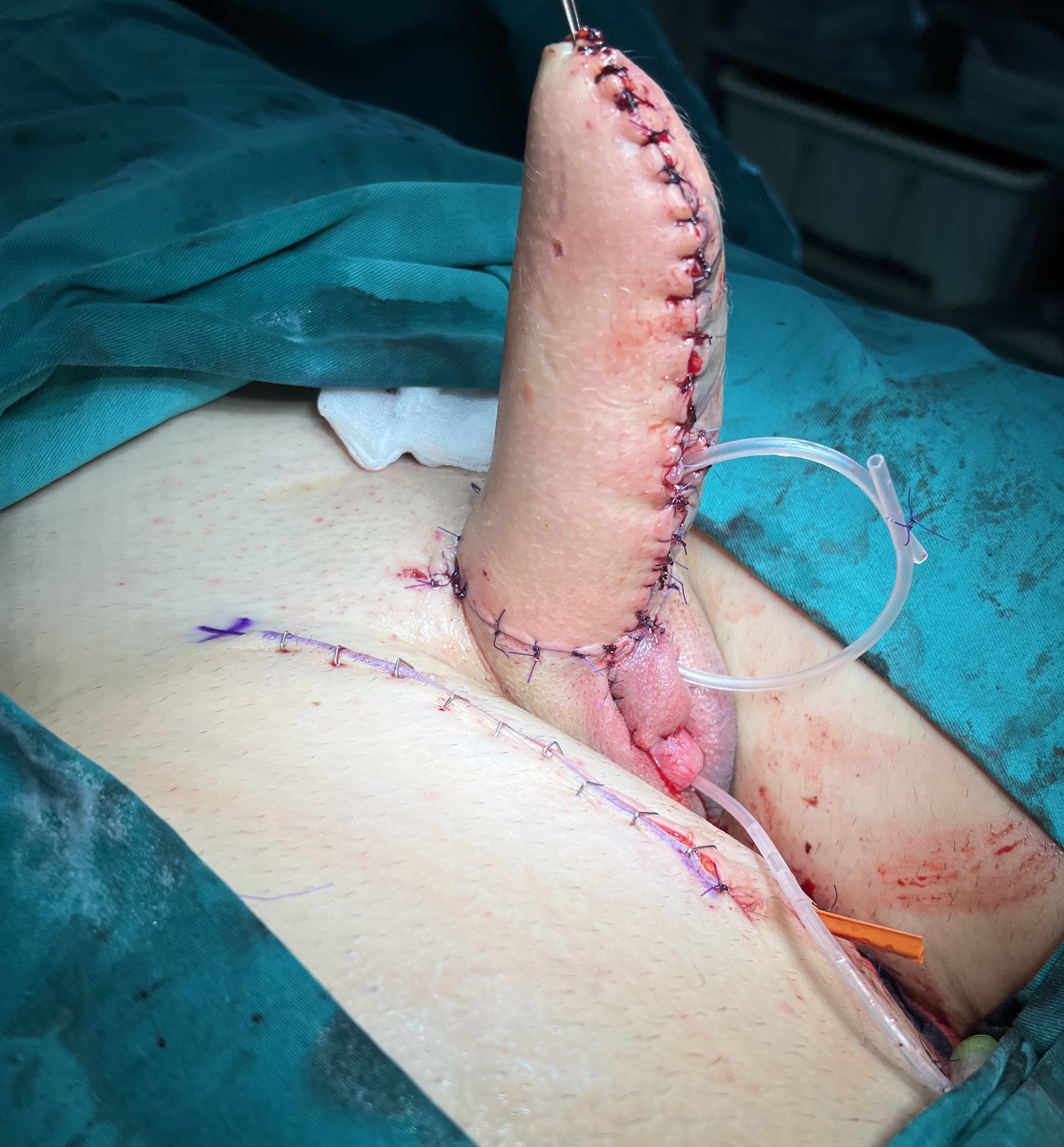 Lateral view. Urethra is lengthened and new opening is located in the proximal part of the neophallus
Lateral view. Urethra is lengthened and new opening is located in the proximal part of the neophallus
CASE 2
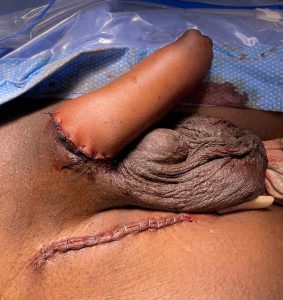
Postoperative outcome after MLD phalloplasty
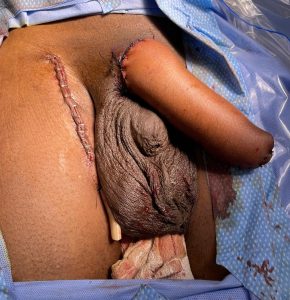
CASE 3
Total MLD phalloplasty – outcome after 12 months
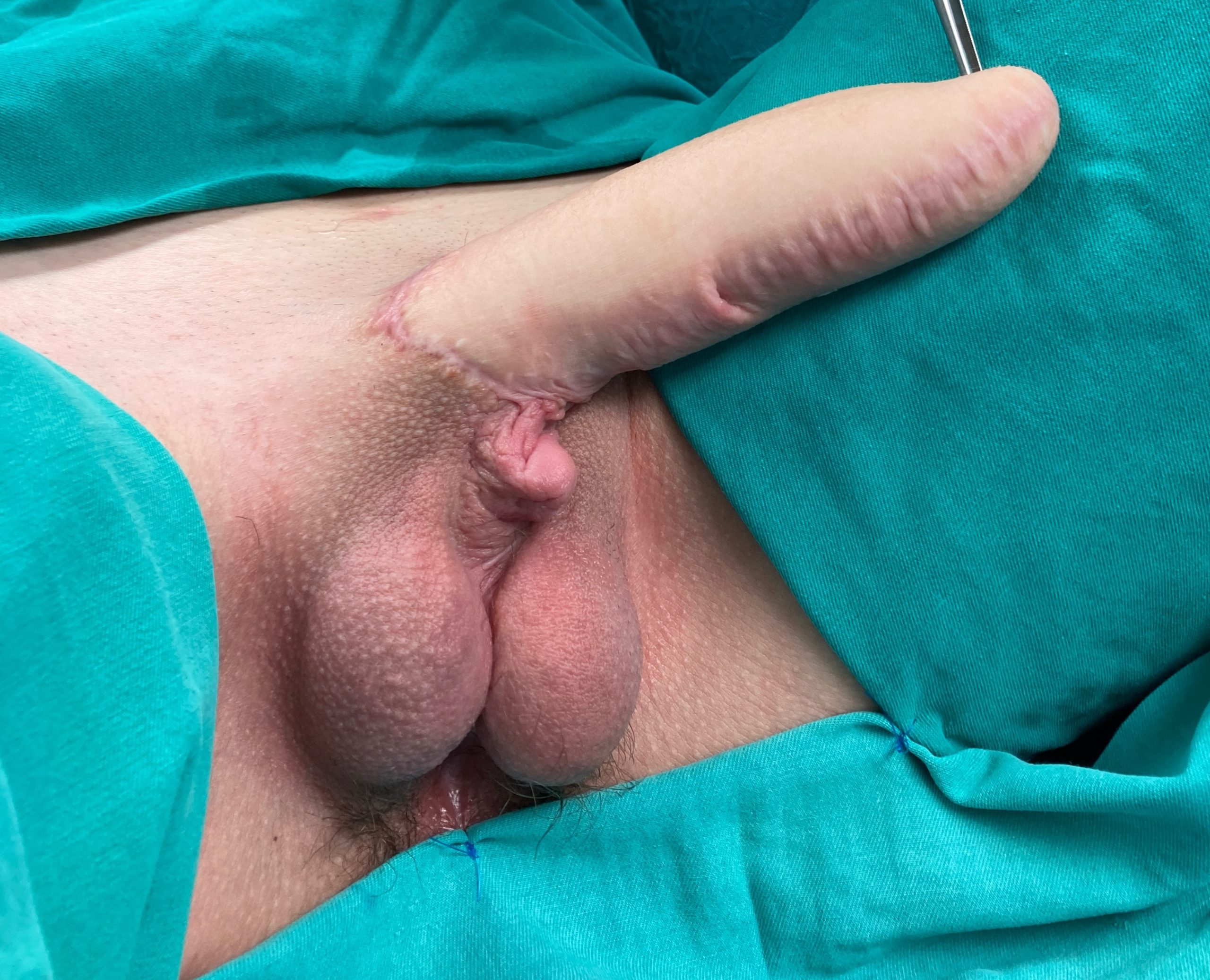
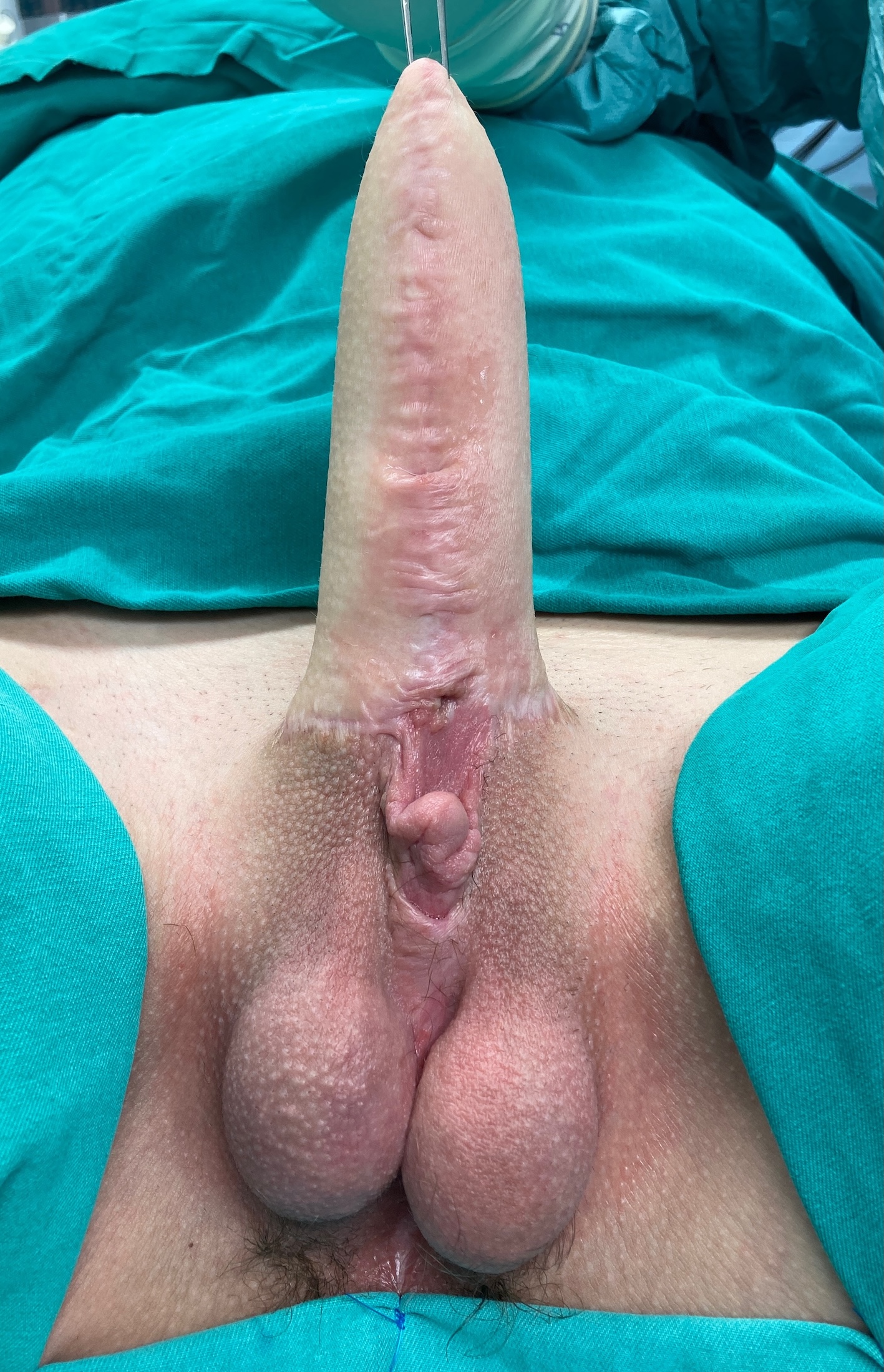
CASE 4
MLD phalloplasty – outcome 4 weeks after surgery


Appearance of the genitalia
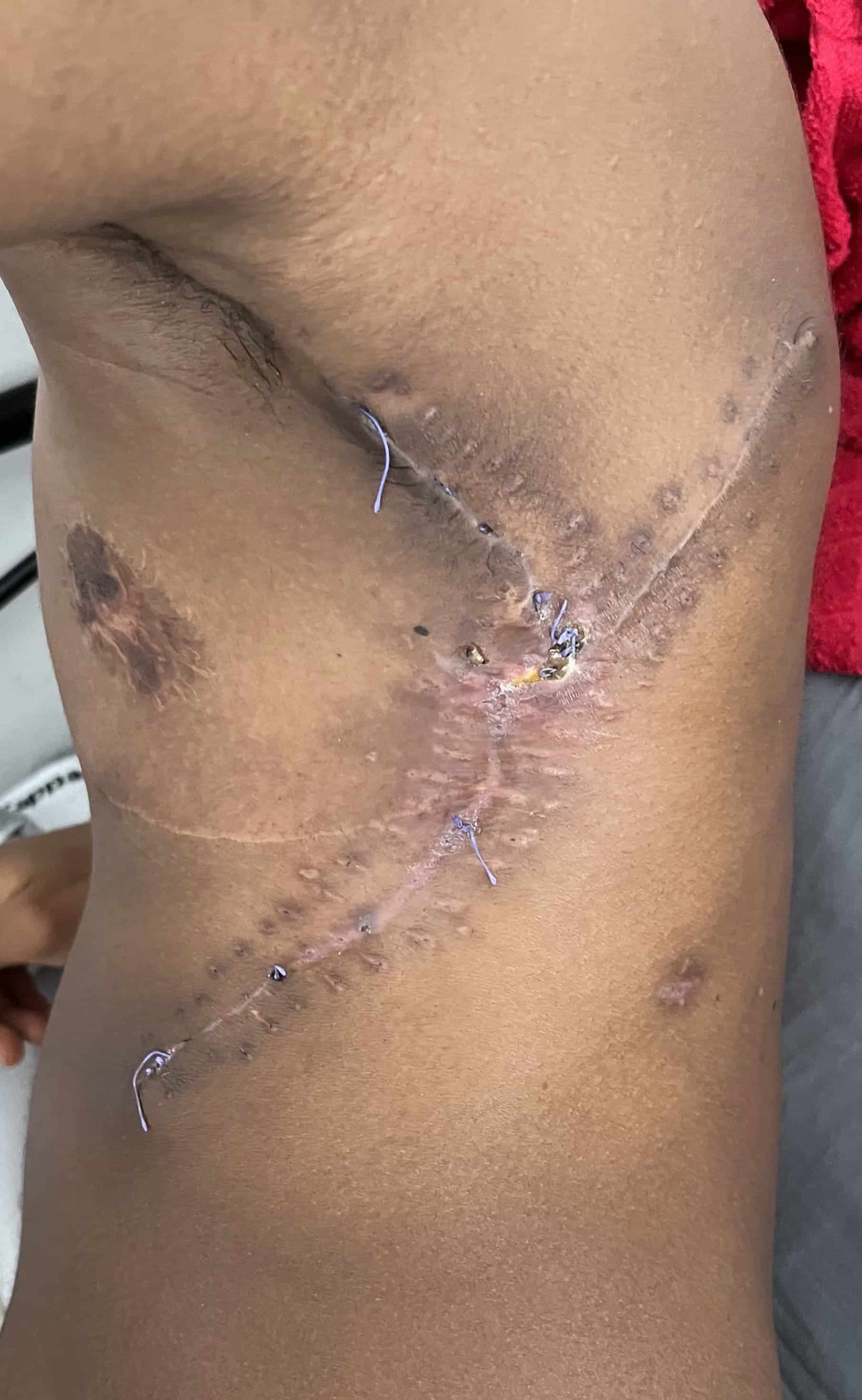
Appearance of the donor area
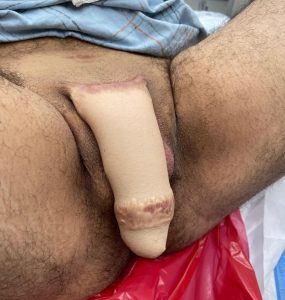
CASE 5
Glansplasty after MLD phalloplasty
Outcome one year after total phalloplasty.

Good aesthetic result after glans reconstruction with skin graft.
CASE 6
Third stage phalloplasty
Postoperative outcome. Glansplasty and urethral lengthening is done.
CASE 7
Good cosmetic outcome one year after complete phalloplasty and glansplasty.

CASE 8
Long-term outcome after MLD phalloplasty.
Good size, shape and position of the neophallus are achieved.
CASE 9
Second stage phalloplasty – glansplasty and penile prosthesis implantation.
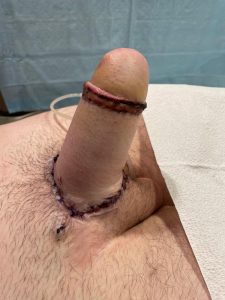
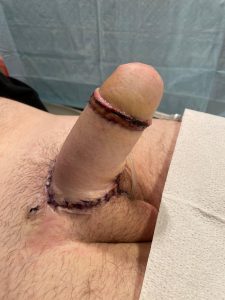
Glans reconstruction is done. Penile prosthesis is placed using dorsal approach.
CASE 10
Final outcome after complete MLD phalloplasty.
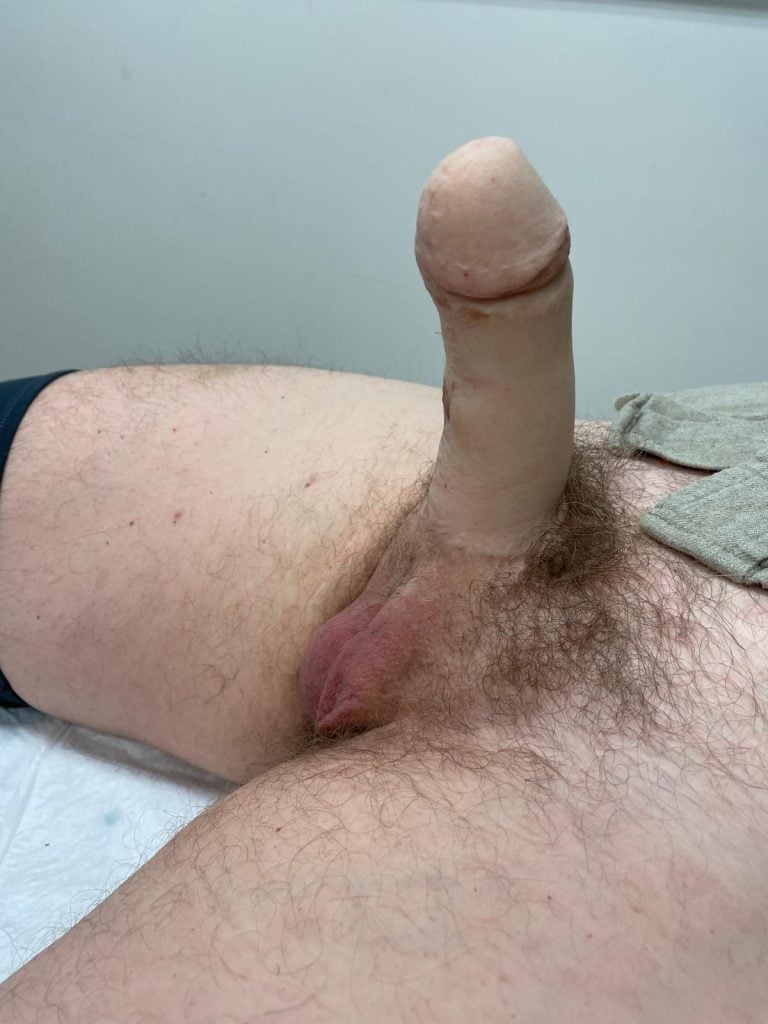
Outcome one year after surgery. Good position of the neophallus. Glansplasty, urethral lengthening and insertion of the penile implant are done.
CASE 11
MLD phalloplasty after metoidioplasty
 Appearance 2 years after metoidioplasty.
Appearance 2 years after metoidioplasty.
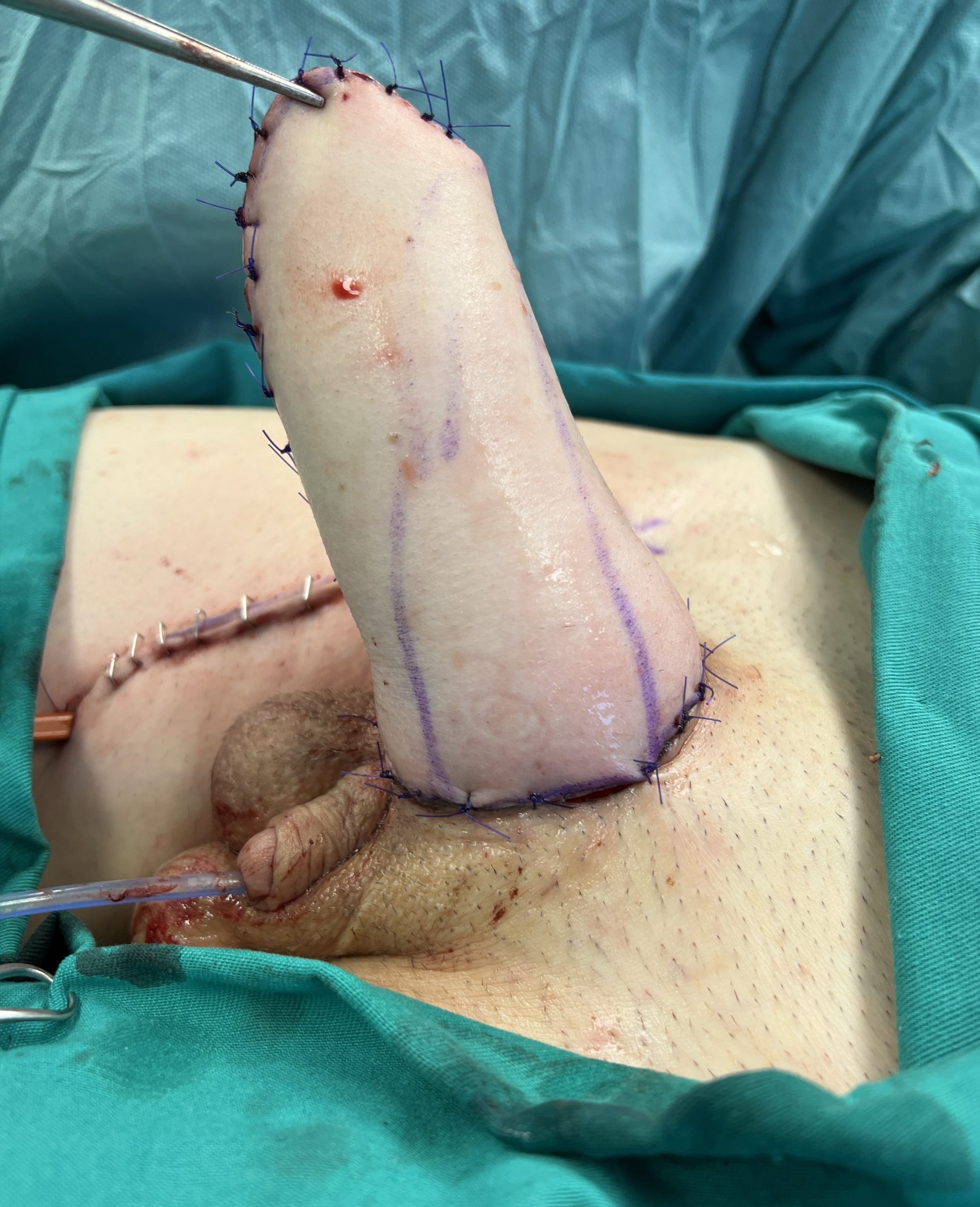
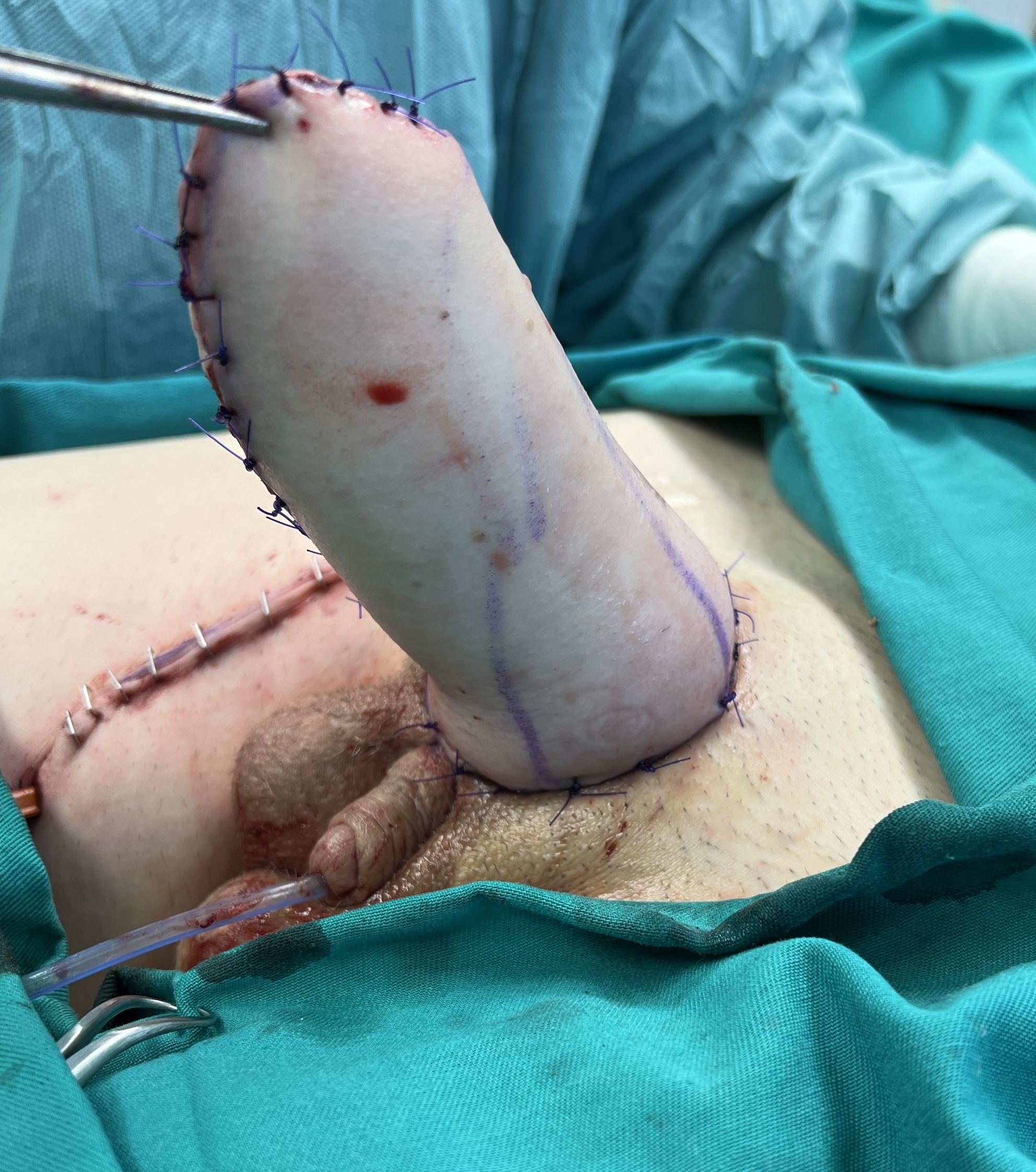 MLD phalloplasty is performed. Good sized and shaped phallus is created.
MLD phalloplasty is performed. Good sized and shaped phallus is created.
CASE 12
MLD phalloplasty after failed phalloplasty elsewhere.
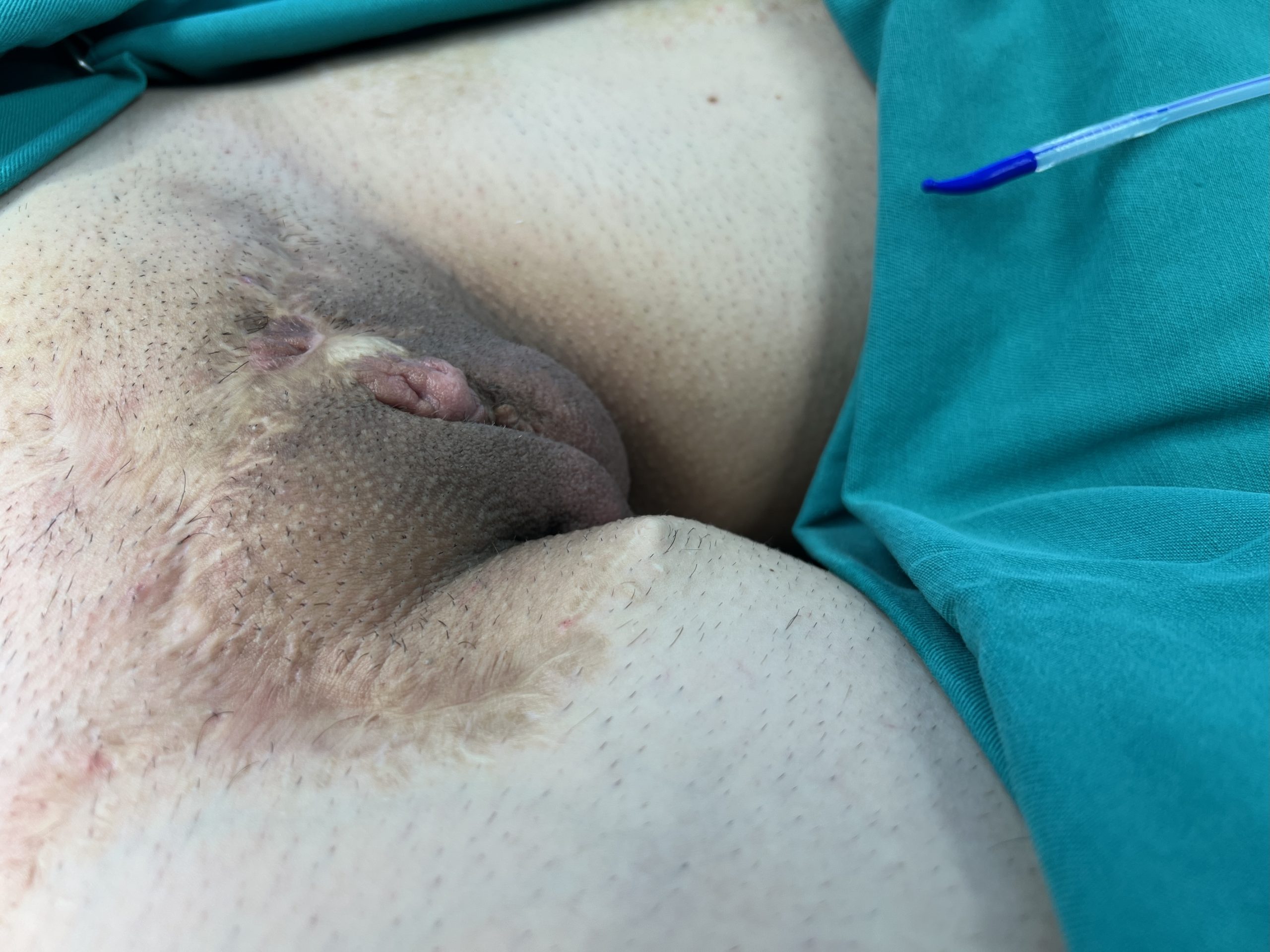
 Appearance of genitalia after failed phalloplasty elsewhere.
Appearance of genitalia after failed phalloplasty elsewhere.
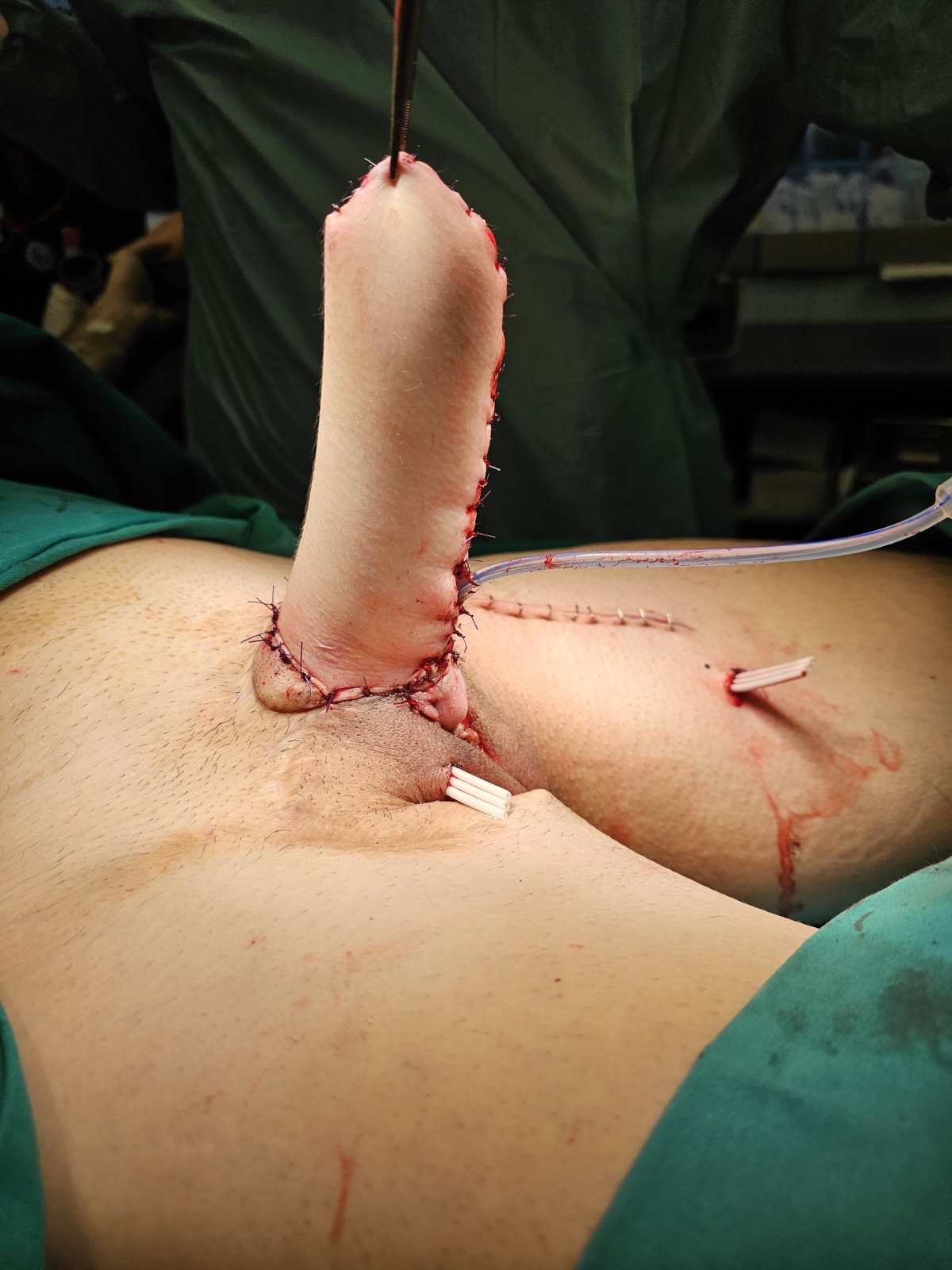 Result after surgery. Good shape and dimensions of the neophallus are achieved.
Result after surgery. Good shape and dimensions of the neophallus are achieved.
CASE 13
Total MLD phalloplasty outcome
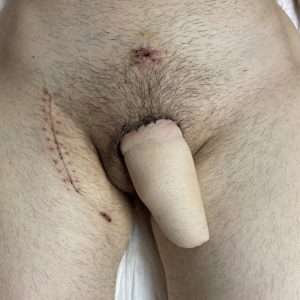

Result 4 weeks after surgery
____________________________________________________________________________________________
CASE 14
Second stage MLD phalloplasty: urethral lengthening, glansplasty, penile prosthesis implantation
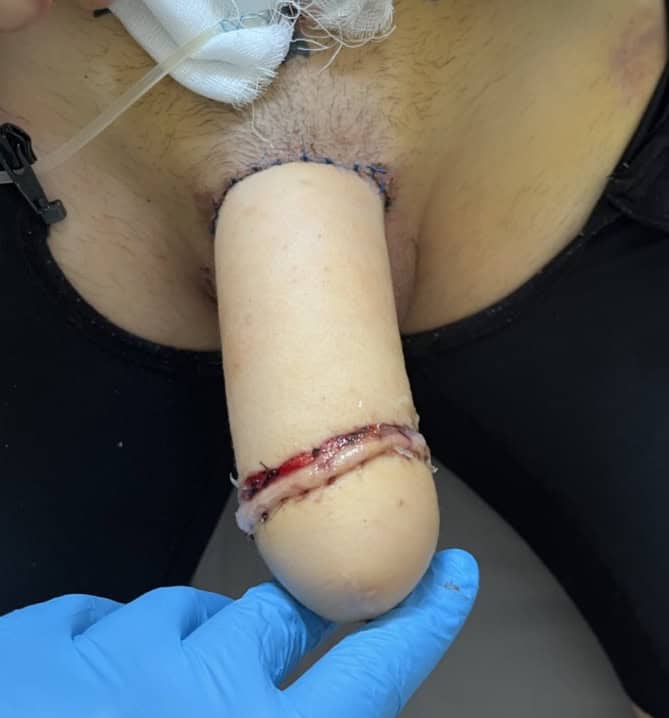
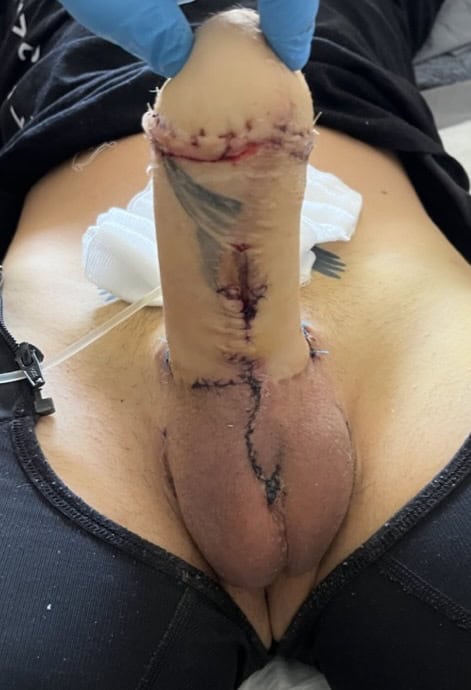
Result 2 weeks after surgery.
______________________________________________________________________________________________
CASE 15
Second stage MLD phalloplasty – glansplasty
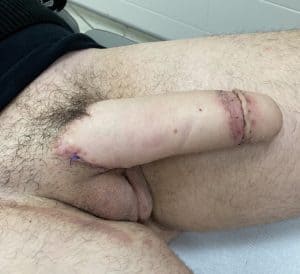
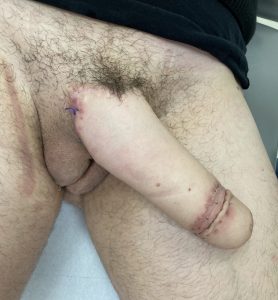
Outcome 4 weeks after surgery
______________________________________________________________________________________________
CASE 16
Second stage MLD phalloplasty – glansplasty and urethral lengthening
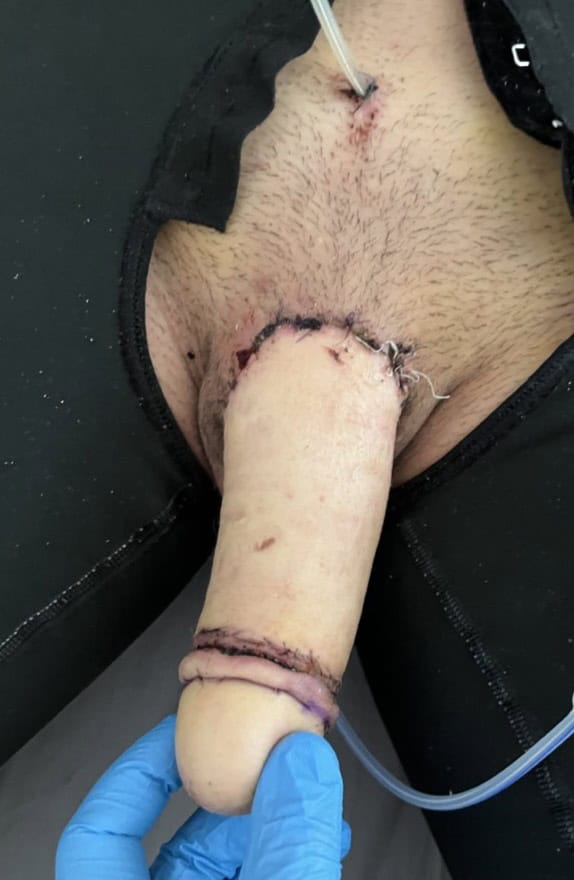


Outcome two weeks after surgery. Suprapubic tube is in place.
CASE 17
Third stage MLD phalloplasty – glansplasty and urethroplasty
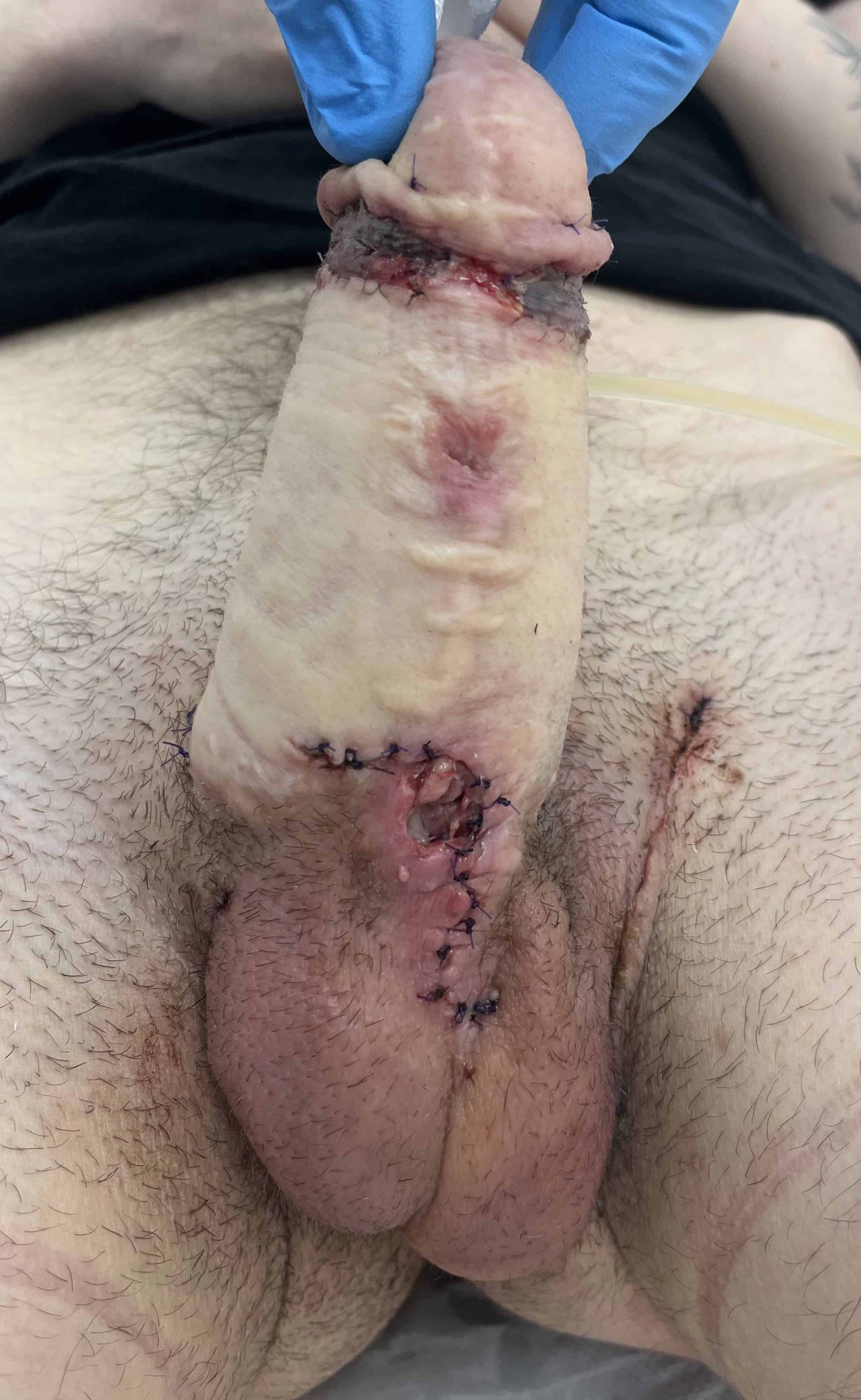
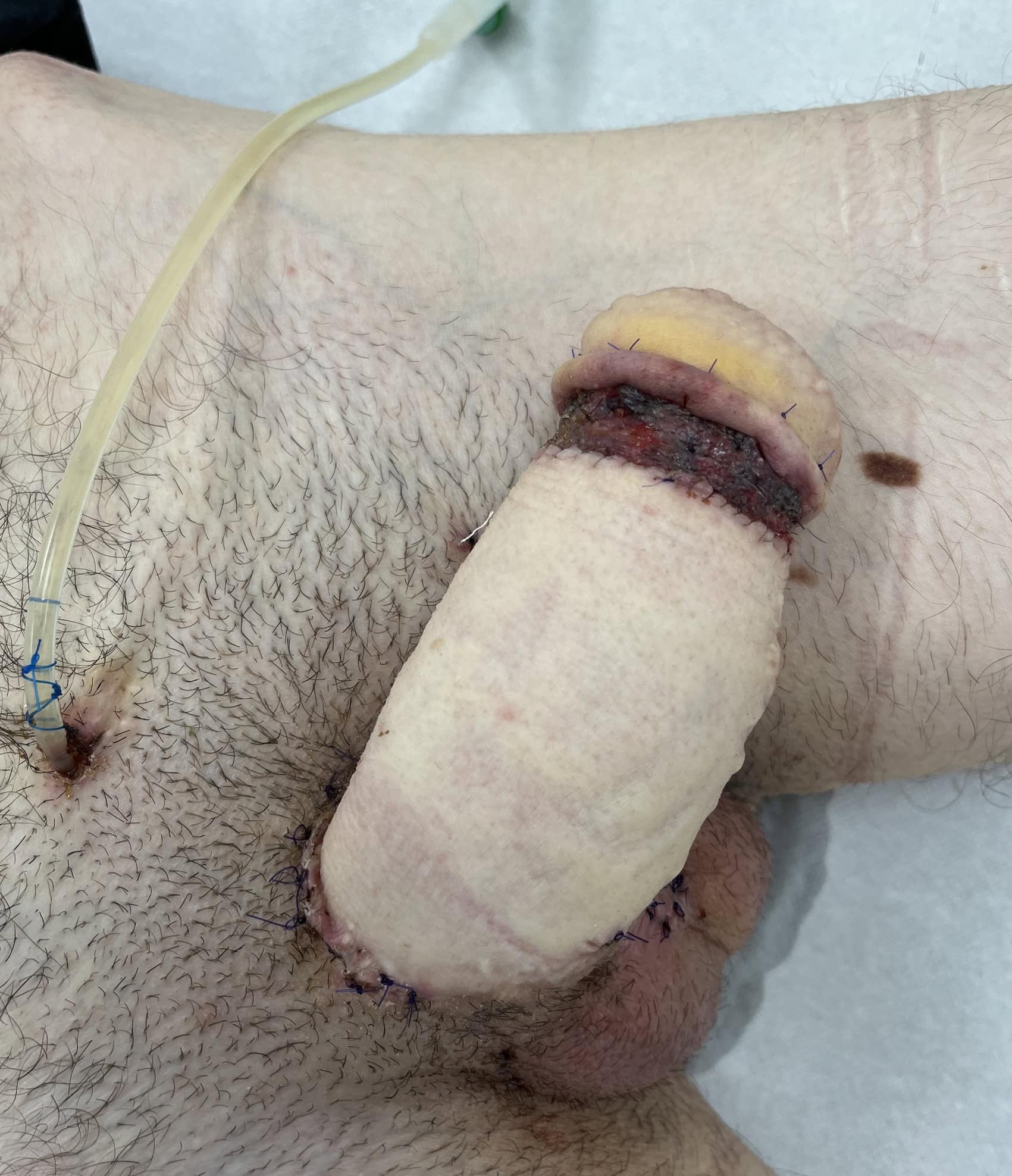
CASE 18
Result after neoglans reconstruction with skin graft
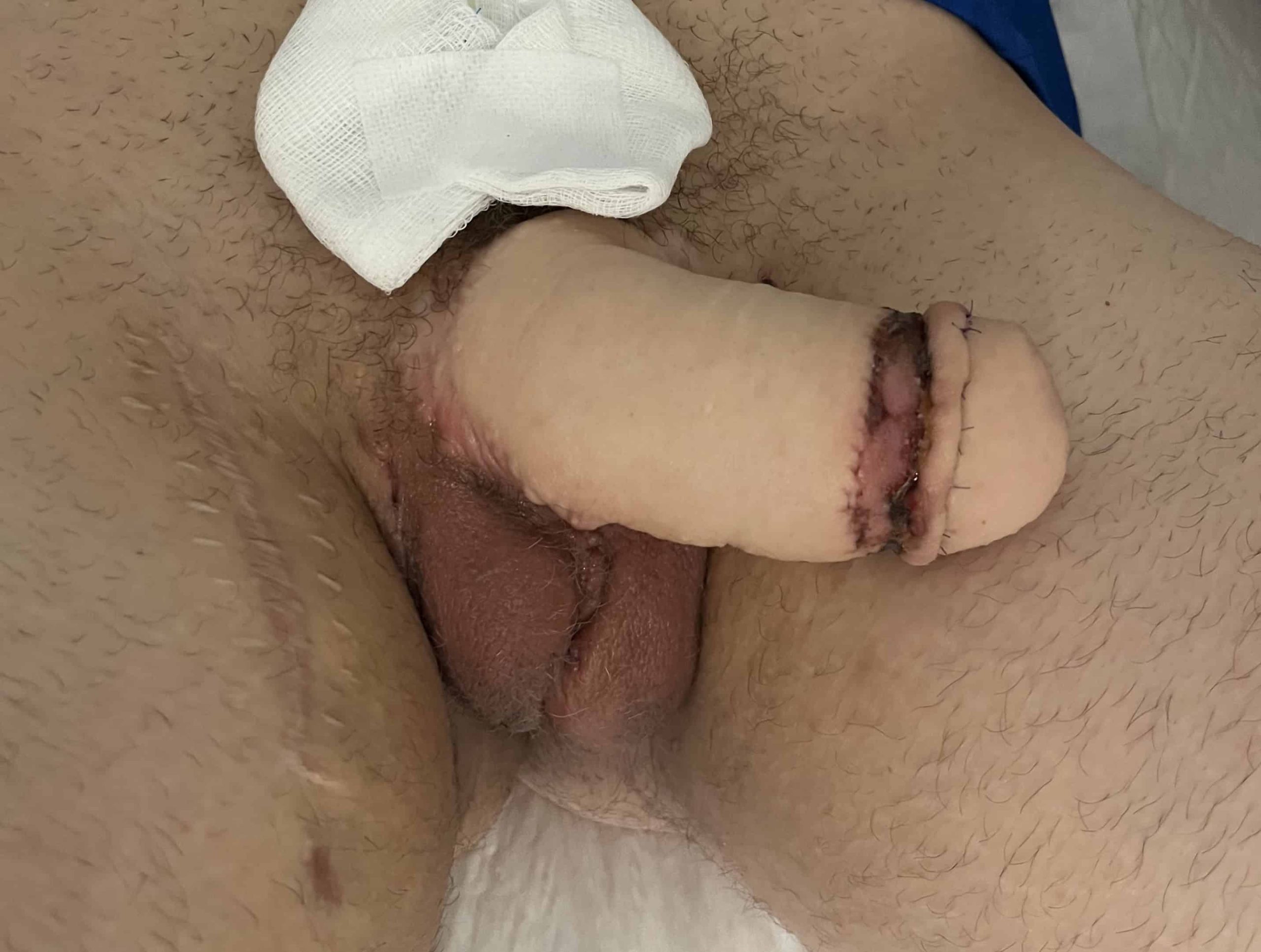
CASE 19
Second stage MLD phalloplasty – urethral lengthening and glans creation

Glans reconstruction with skin graft is performed.
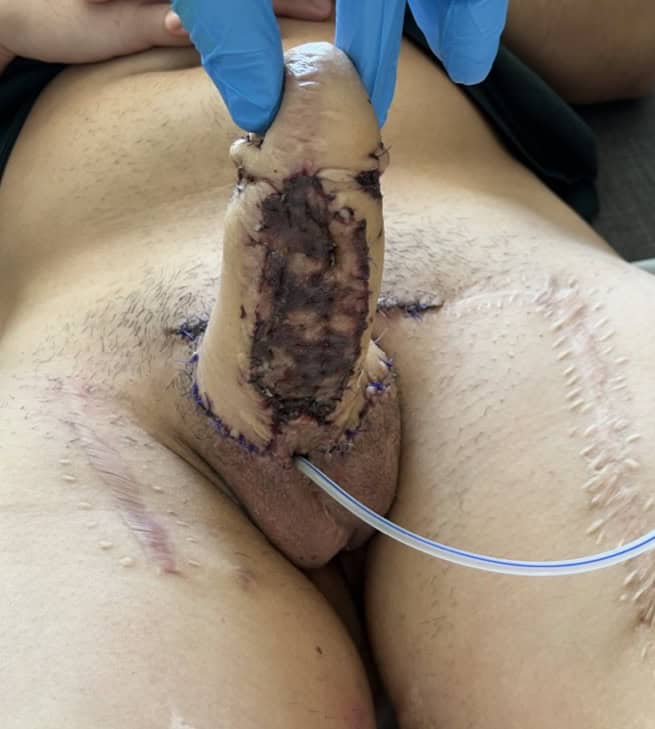

Skin graft is placed and fixed as a first stage of urethral lengthening.
CASE 20
Result after complete reconstruction.

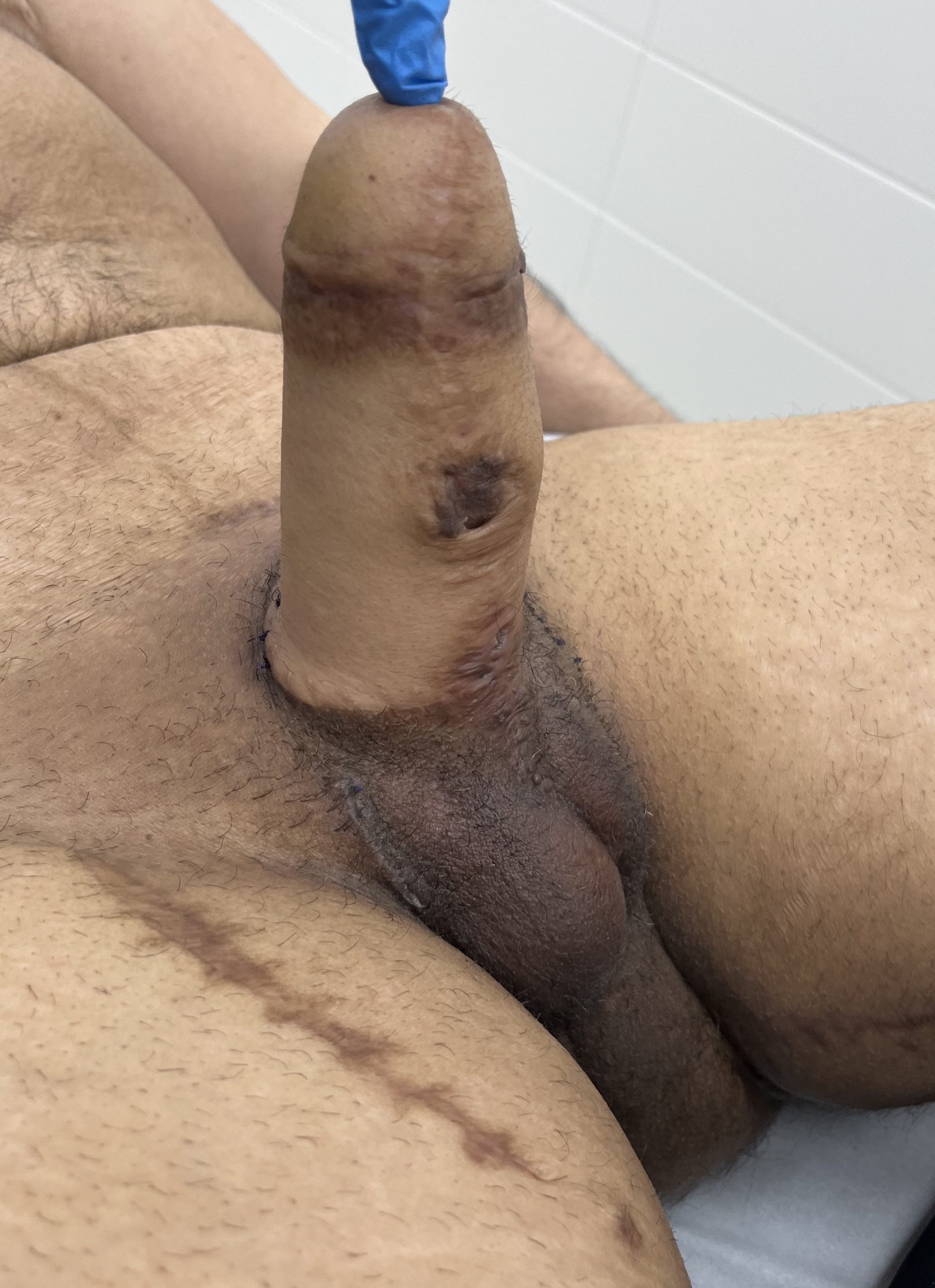
Result after 3 stage reconstruction: MLD phalloplasty, urethral lengthening, penile prosthesis implantation and glansplasty
CASE 21
Result one year after total phalloplasty

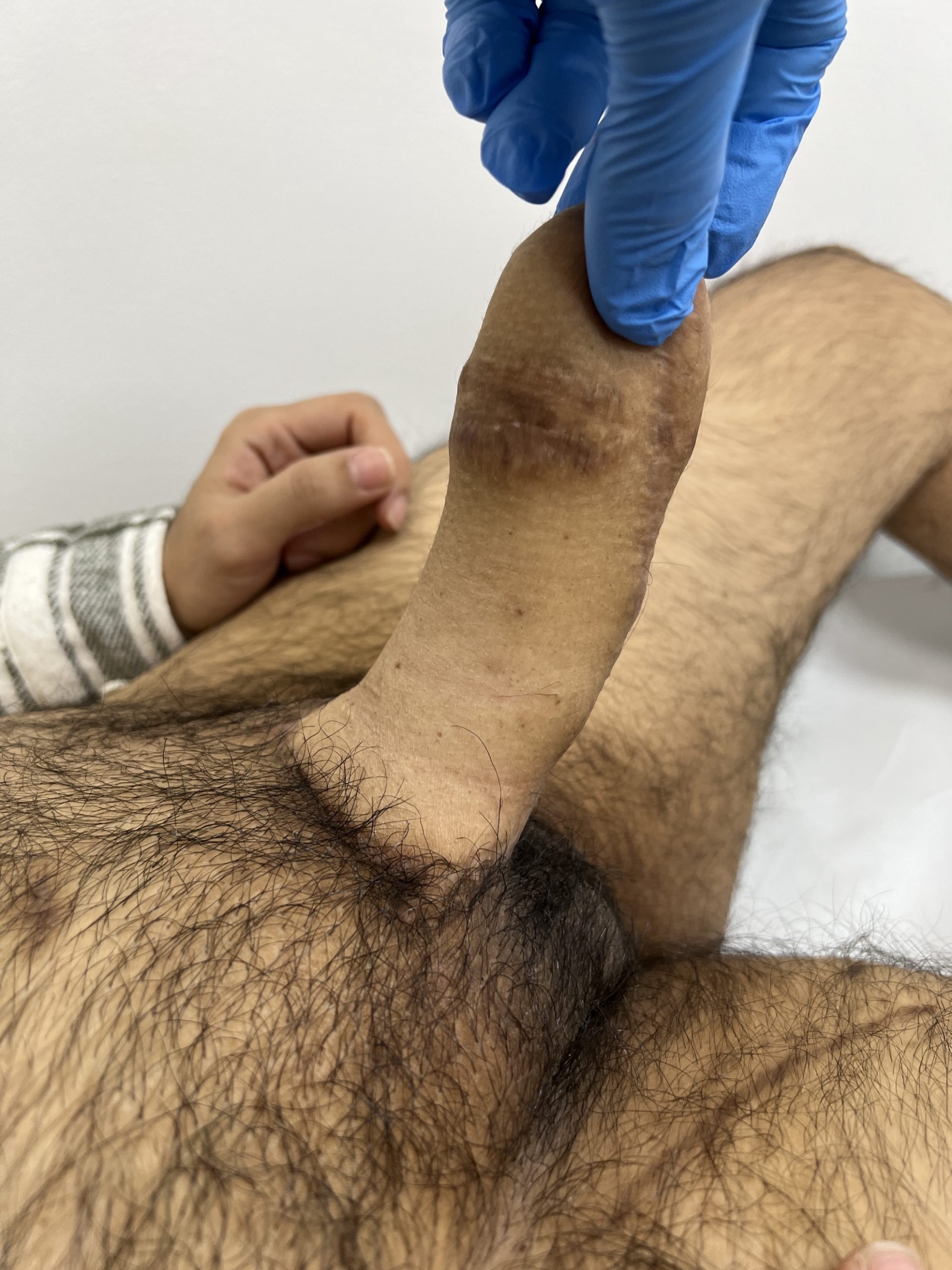
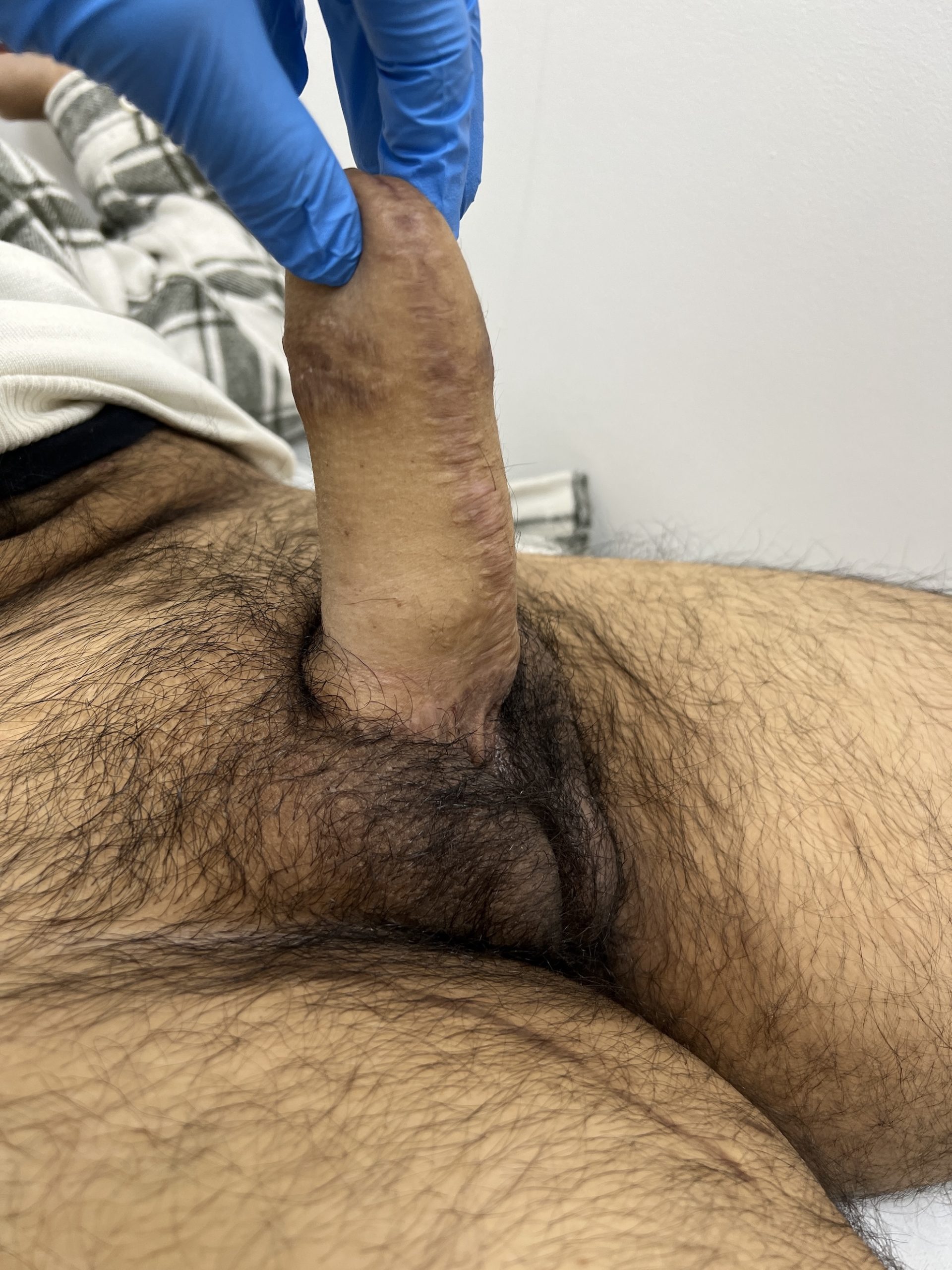
CASE 22
Outcome after complete reconstruction

 Result after 3 stage reconstruction: MLD phalloplasty, urethral lengthening, penile prosthesis implantation and glansplasty
Result after 3 stage reconstruction: MLD phalloplasty, urethral lengthening, penile prosthesis implantation and glansplasty
CASE 23
Abdominal phalloplasty
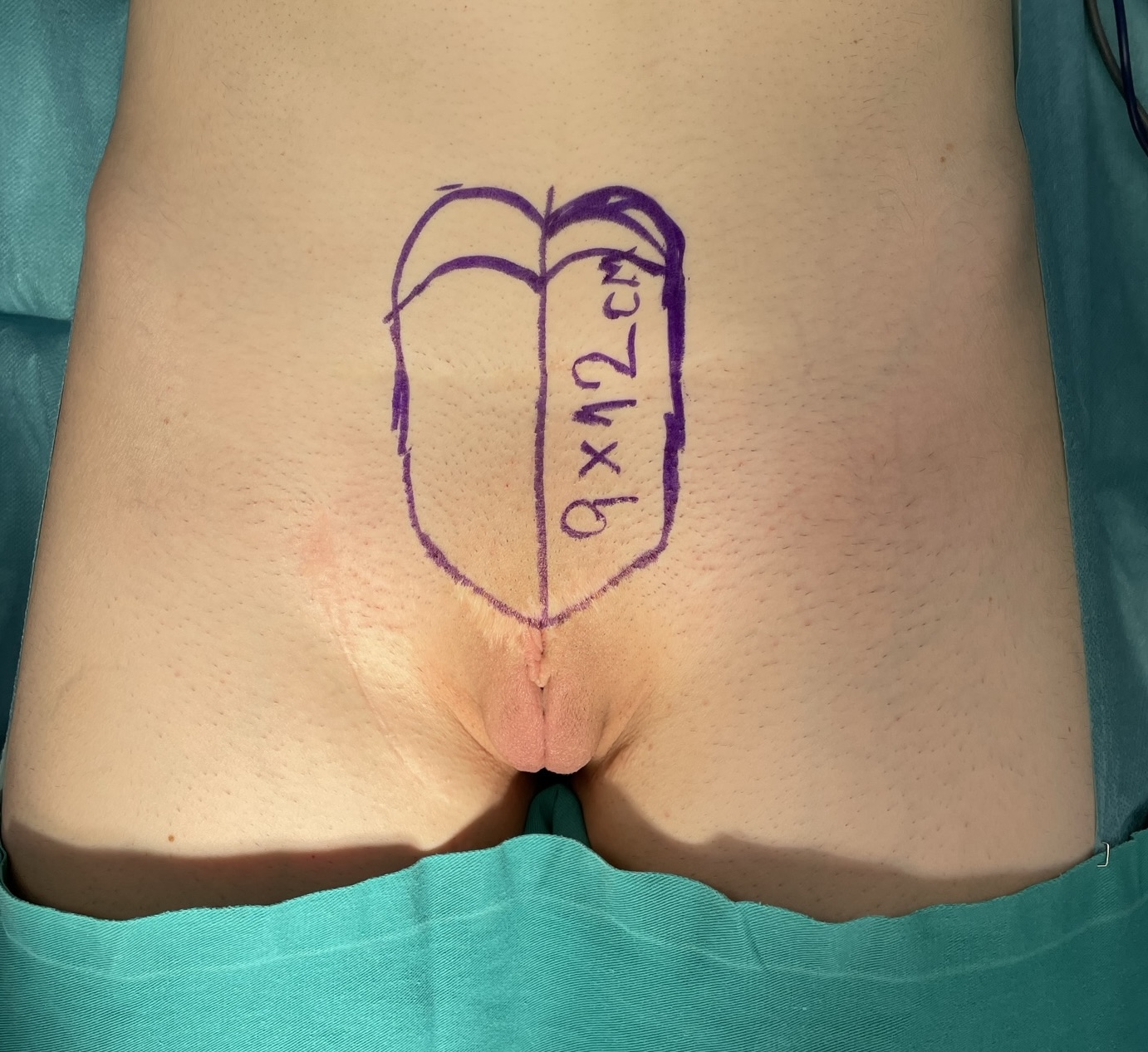
Preoperative appearance. A 9x12cm suprapubic flap is designed.
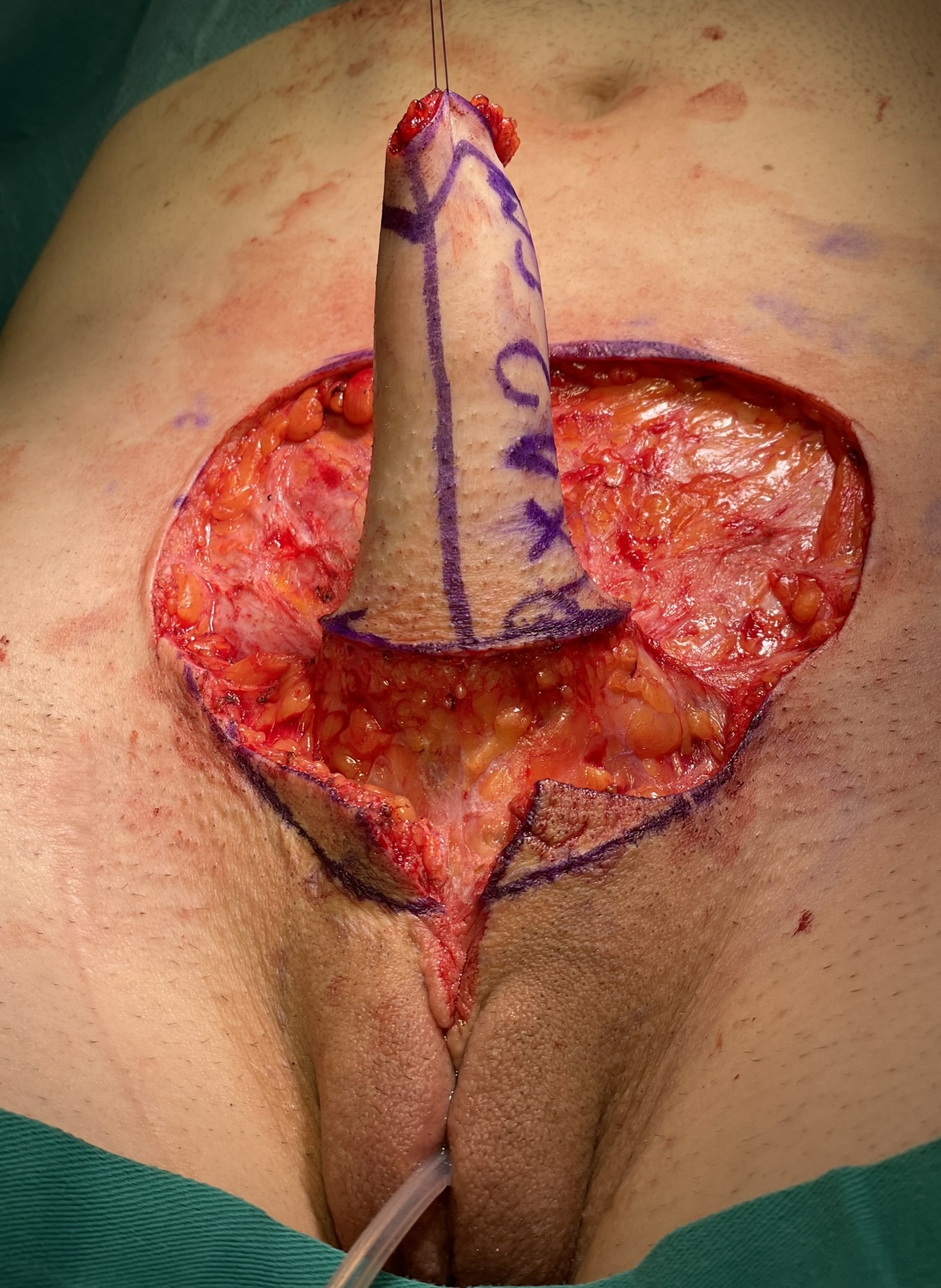
Pedicled abdominal flap is harvested and tubularised.
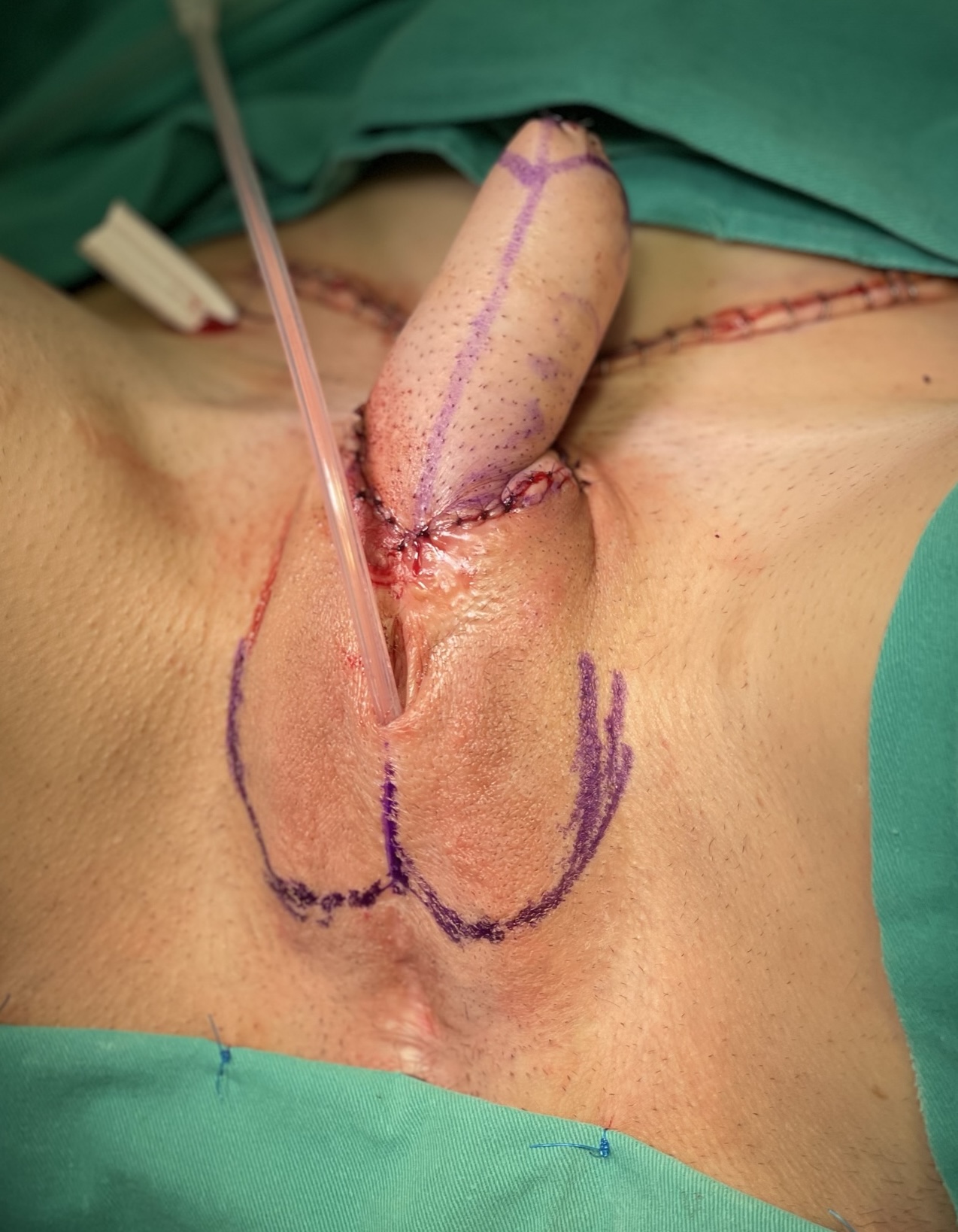
Donor area is closed by direct approximation. Scrotum is designed.
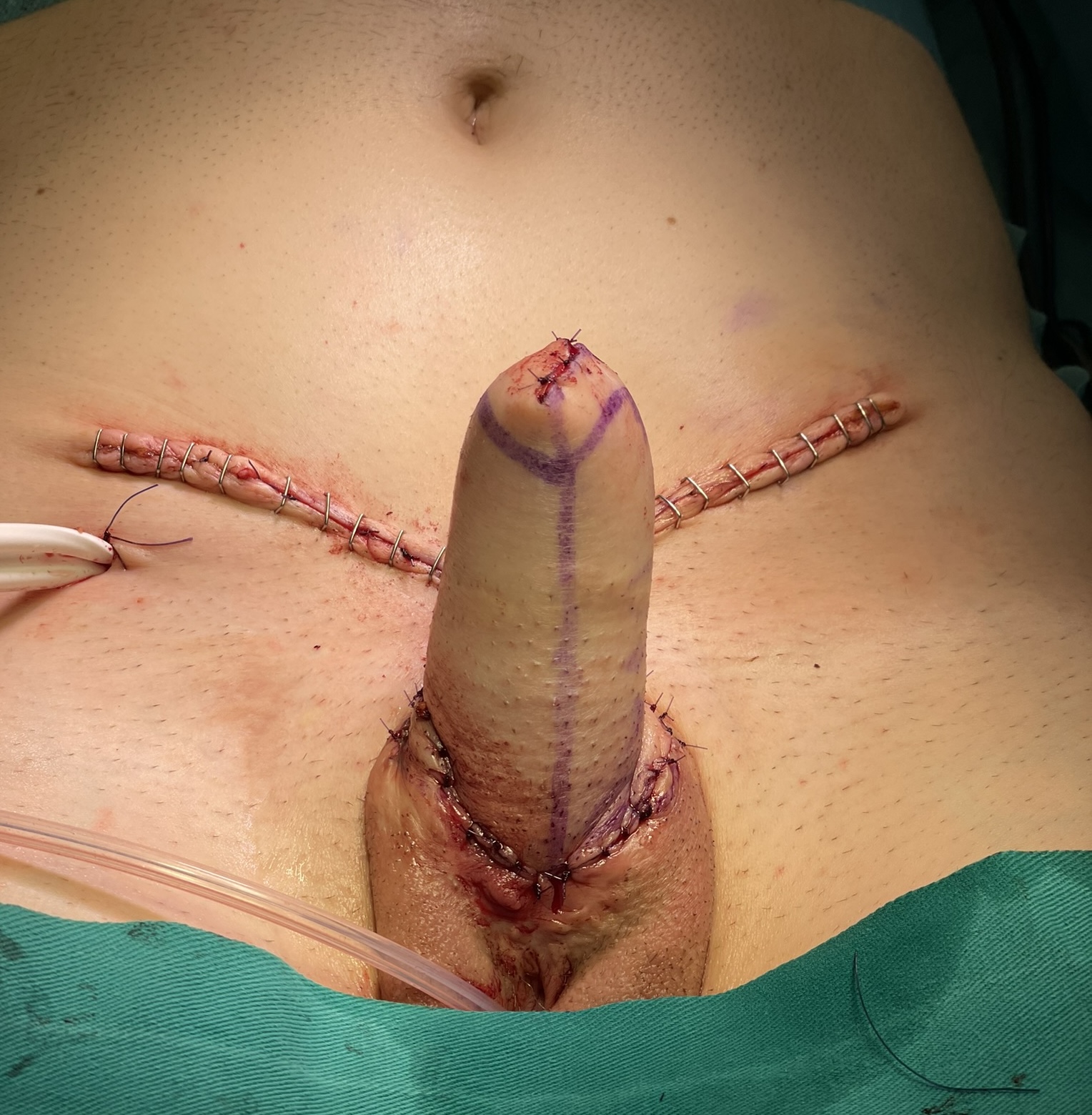
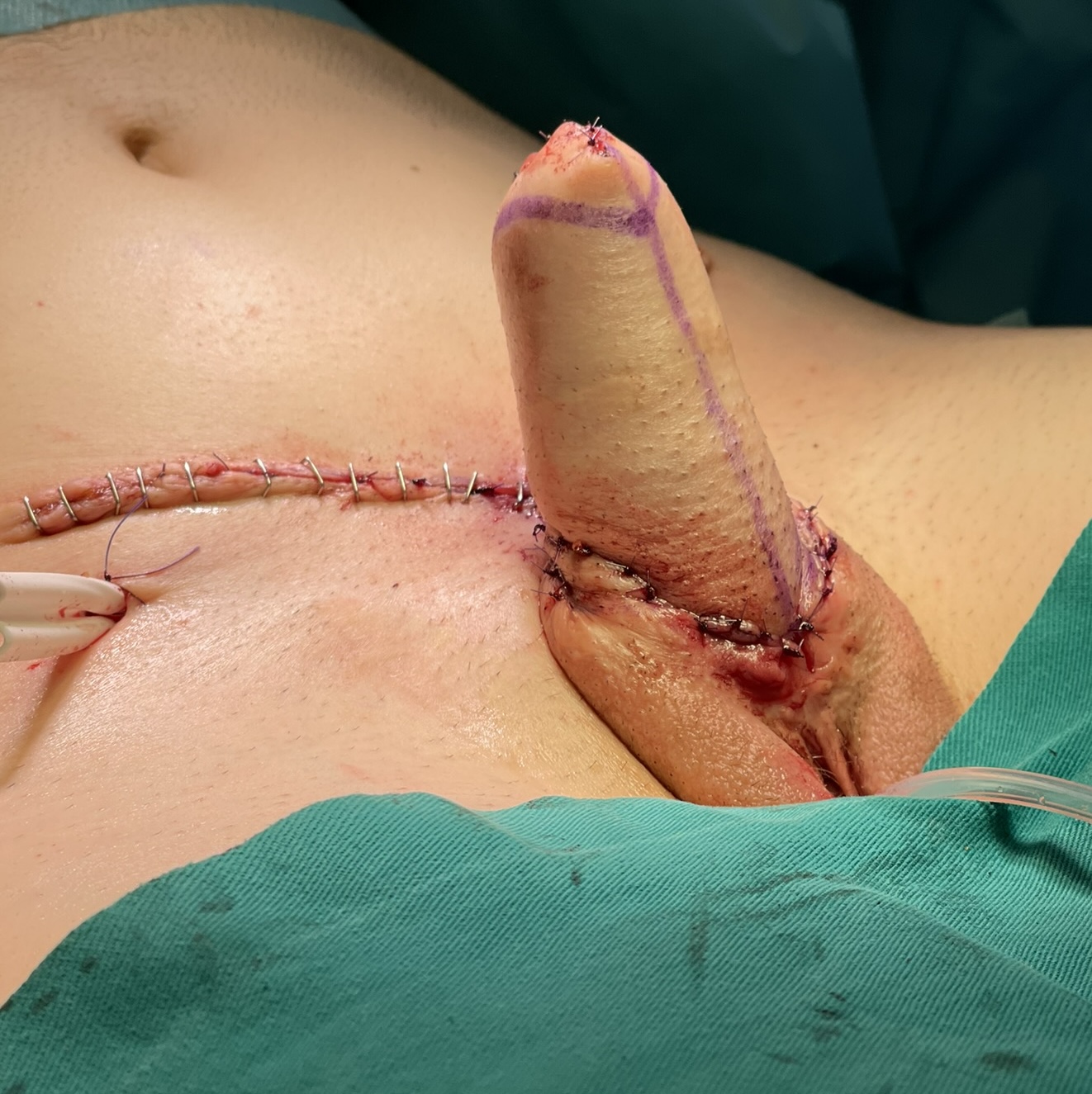
Scrotoplasty is done. Good appearance is achieved.
CASE 24
Abdominal phalloplasty
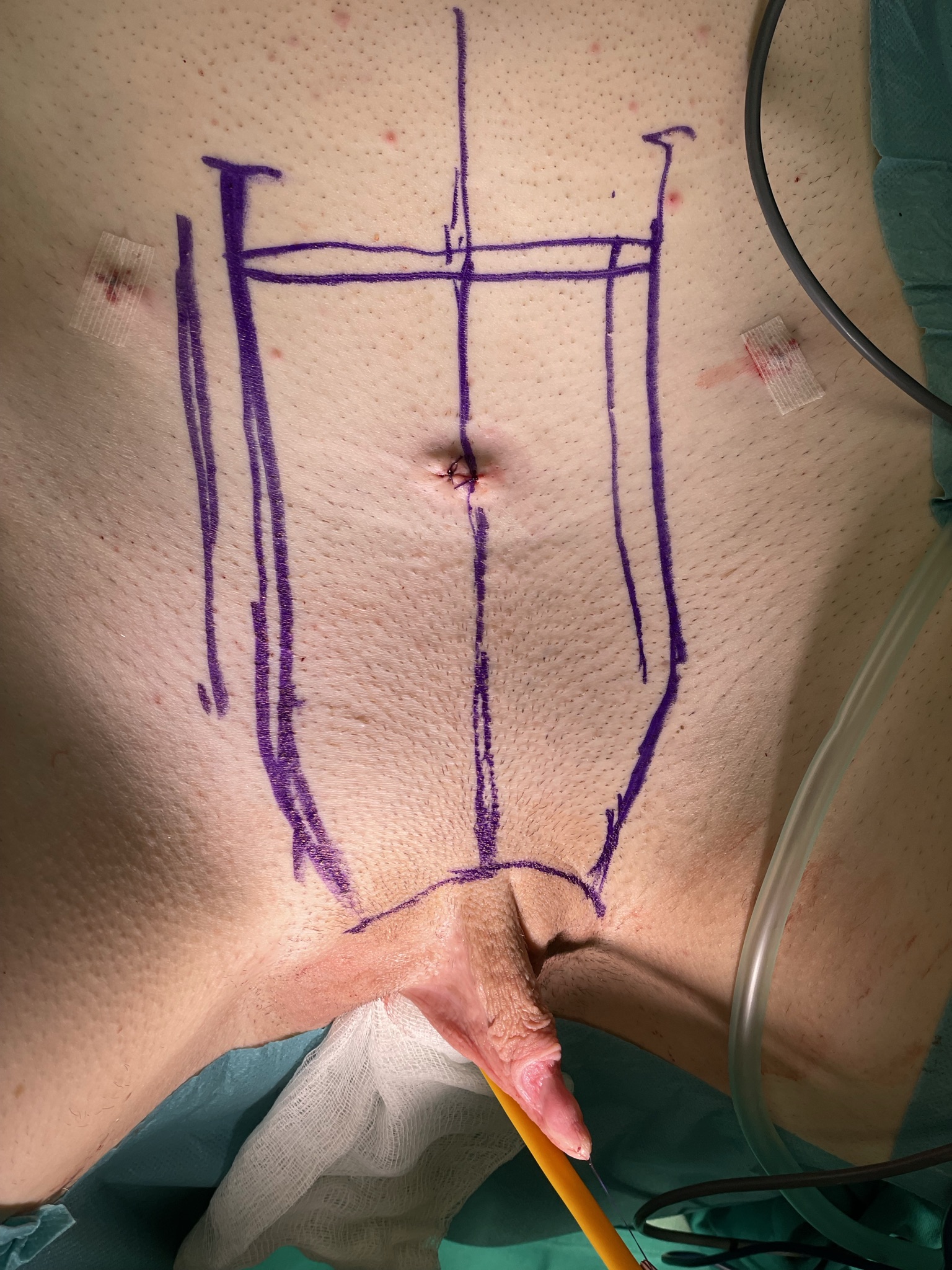
Laparoscopic hysterectomy in done. Abdominal flap is designed.
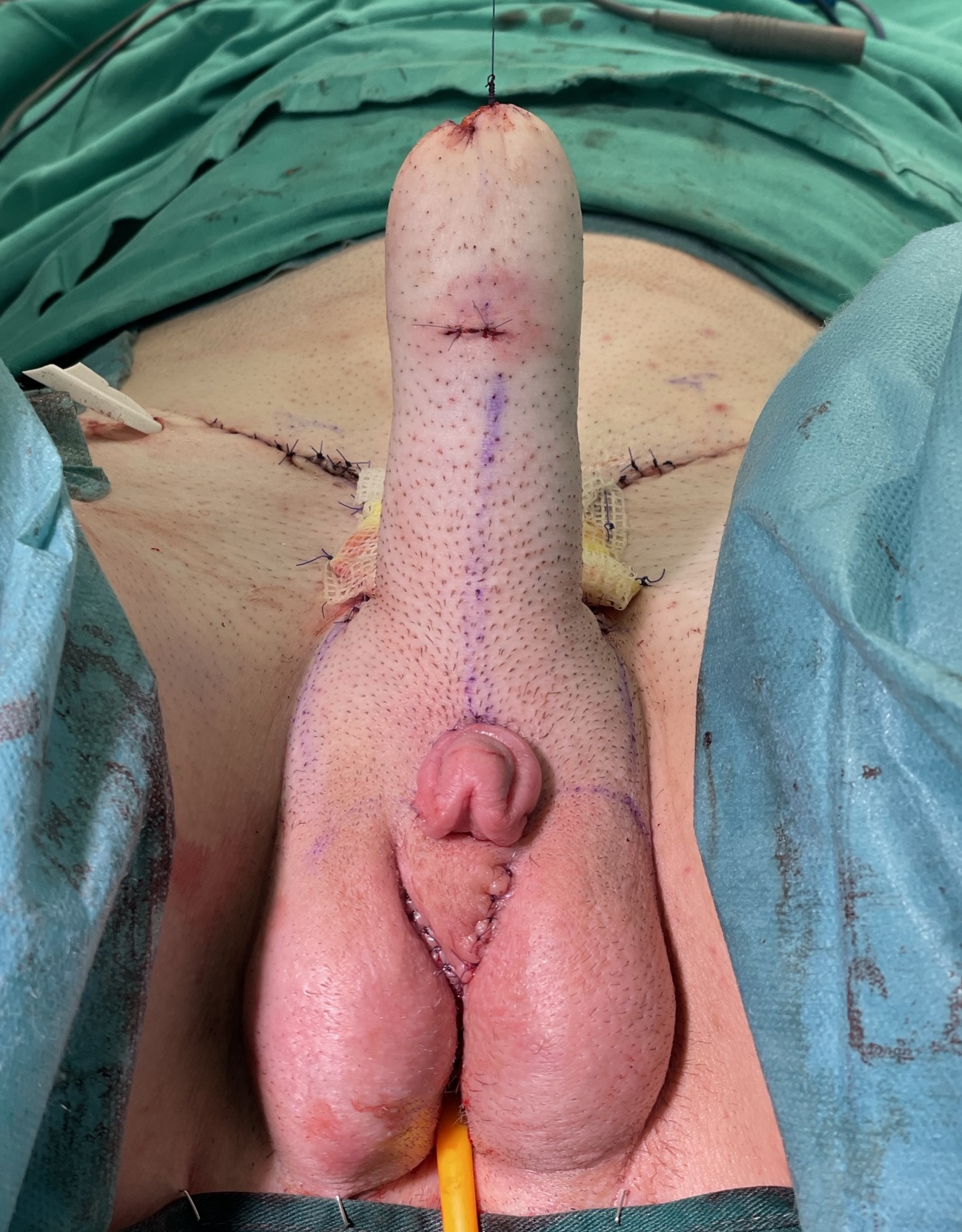
Outcome after surgery. Scrotoplasty is performed, and clitoral glans is fixed at the base of the neophallus.
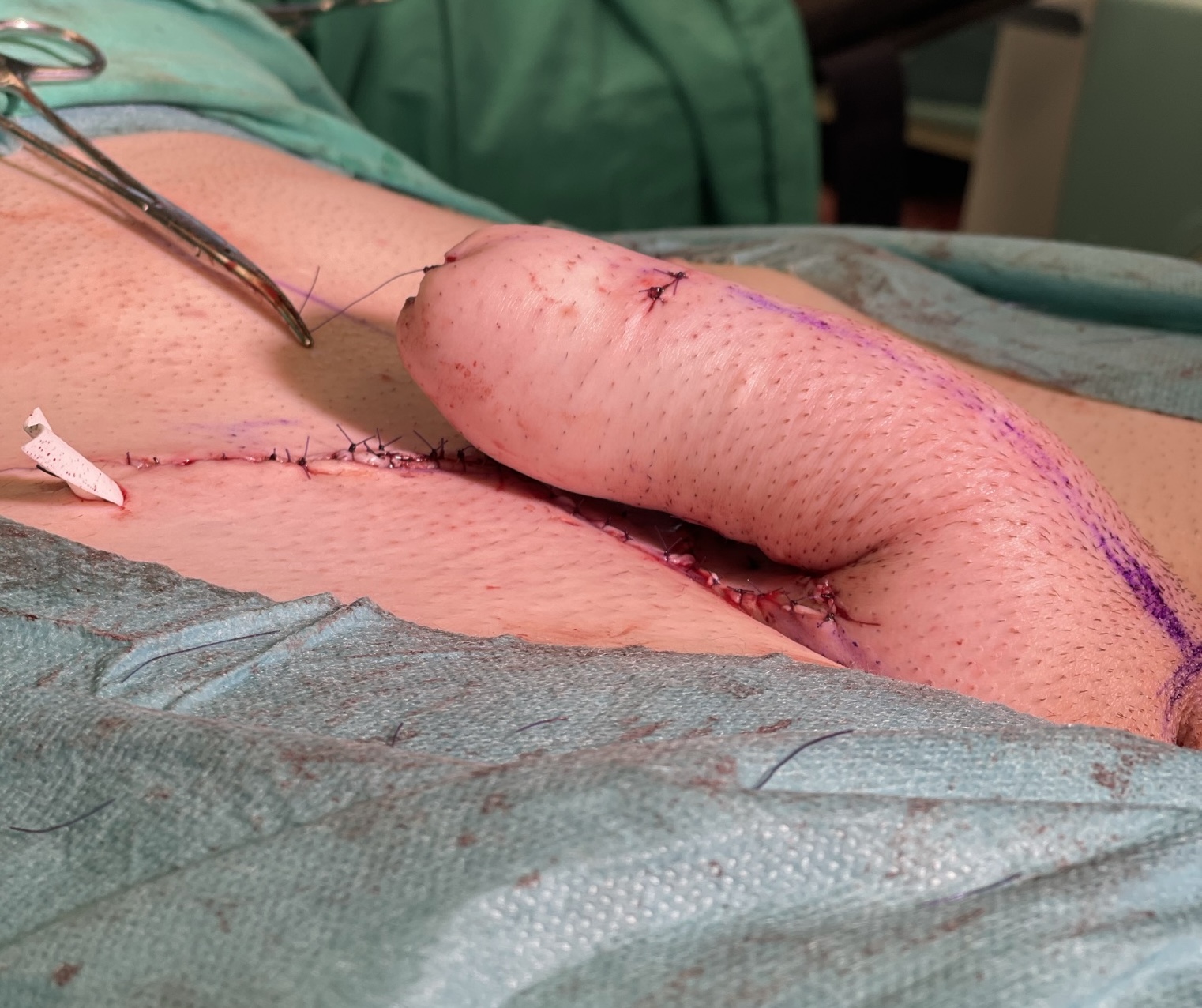
Result after surgery – lateral view.

Outcome six months after surgery.
CASE 25
Abdominal phalloplasty
Preoperative appearance with flap design.
Harvesting of the pedicled flap.
Postoperative outcome. Urethral lengthening is done to the proximal part of the neophallus.
Result after surgery. Clitoral glans is buried under the skin, according to patient’s preference.
CASE 26
Glansplasty after abdominal phalloplasty
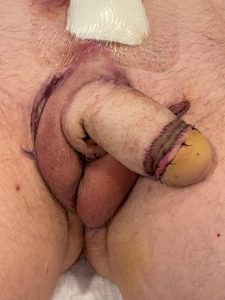
CASE 27
Abdominal phalloplasty – penile prosthesis implantation
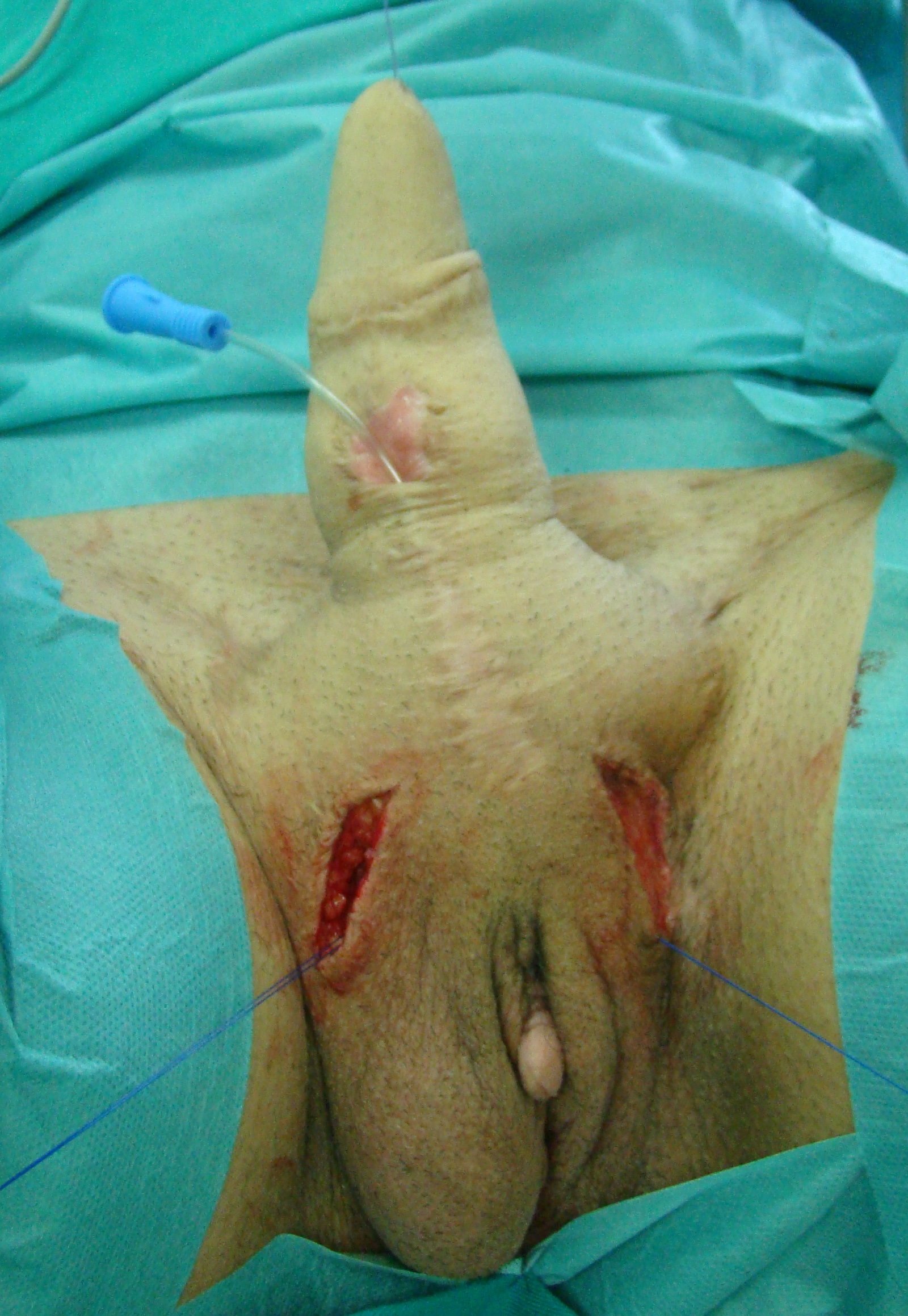
Outcome one year after abdominal phalloplasty, urethral lengthening and glansplasty. Sutures are placed at the pubic bone for prosthesis fixation.
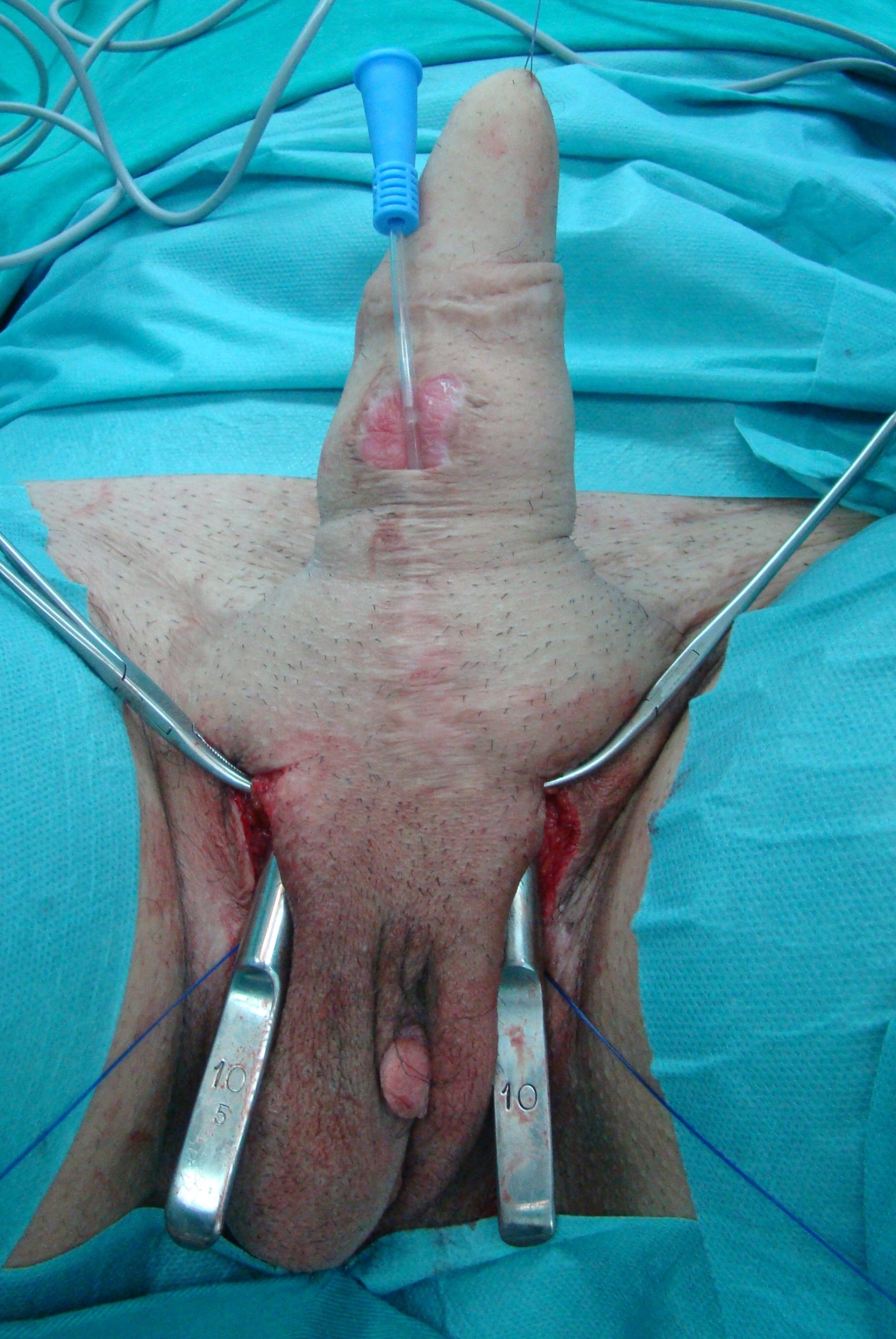
Space for two cylinder prosthesis insertion is created into the neophallus.
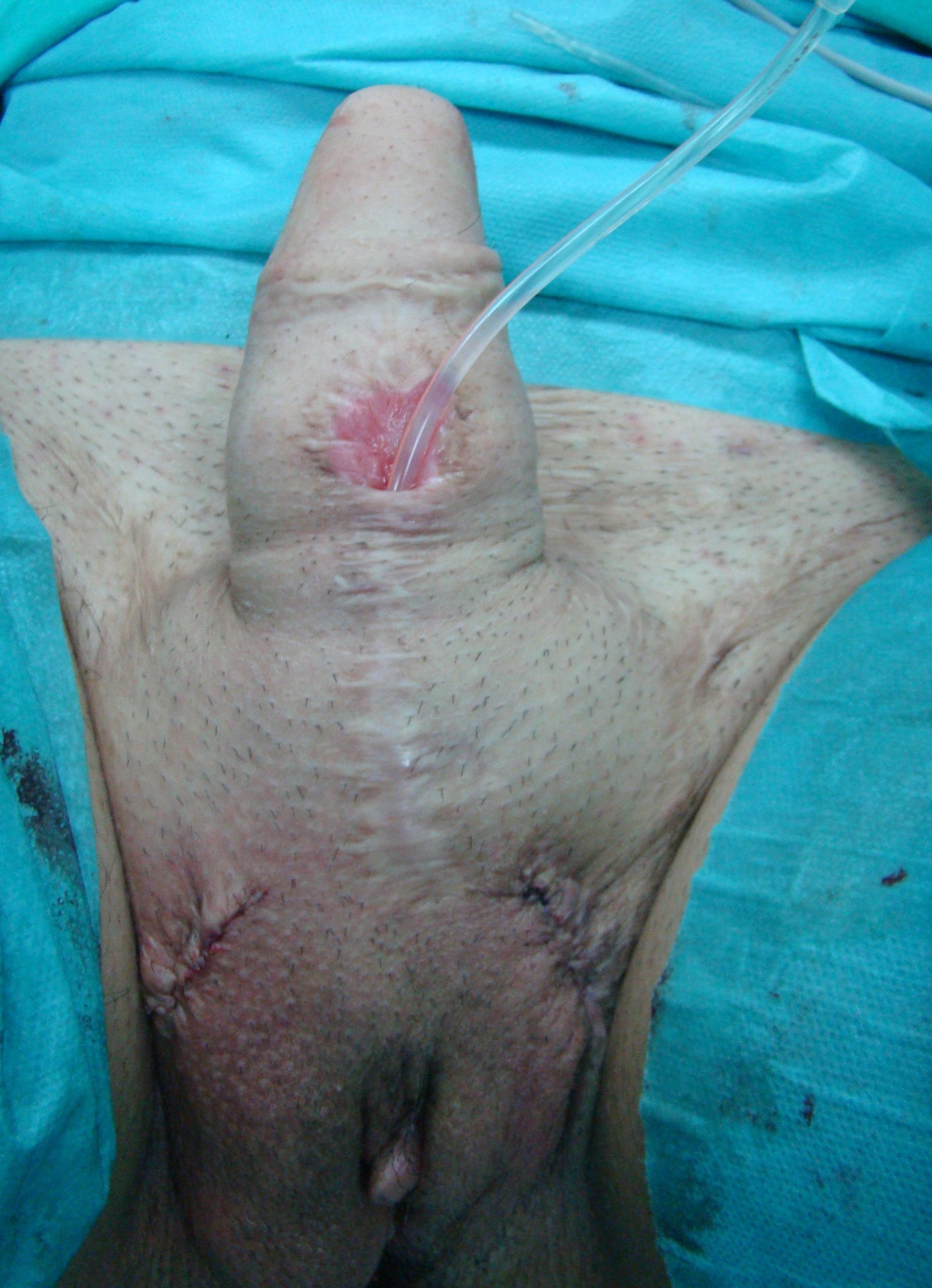
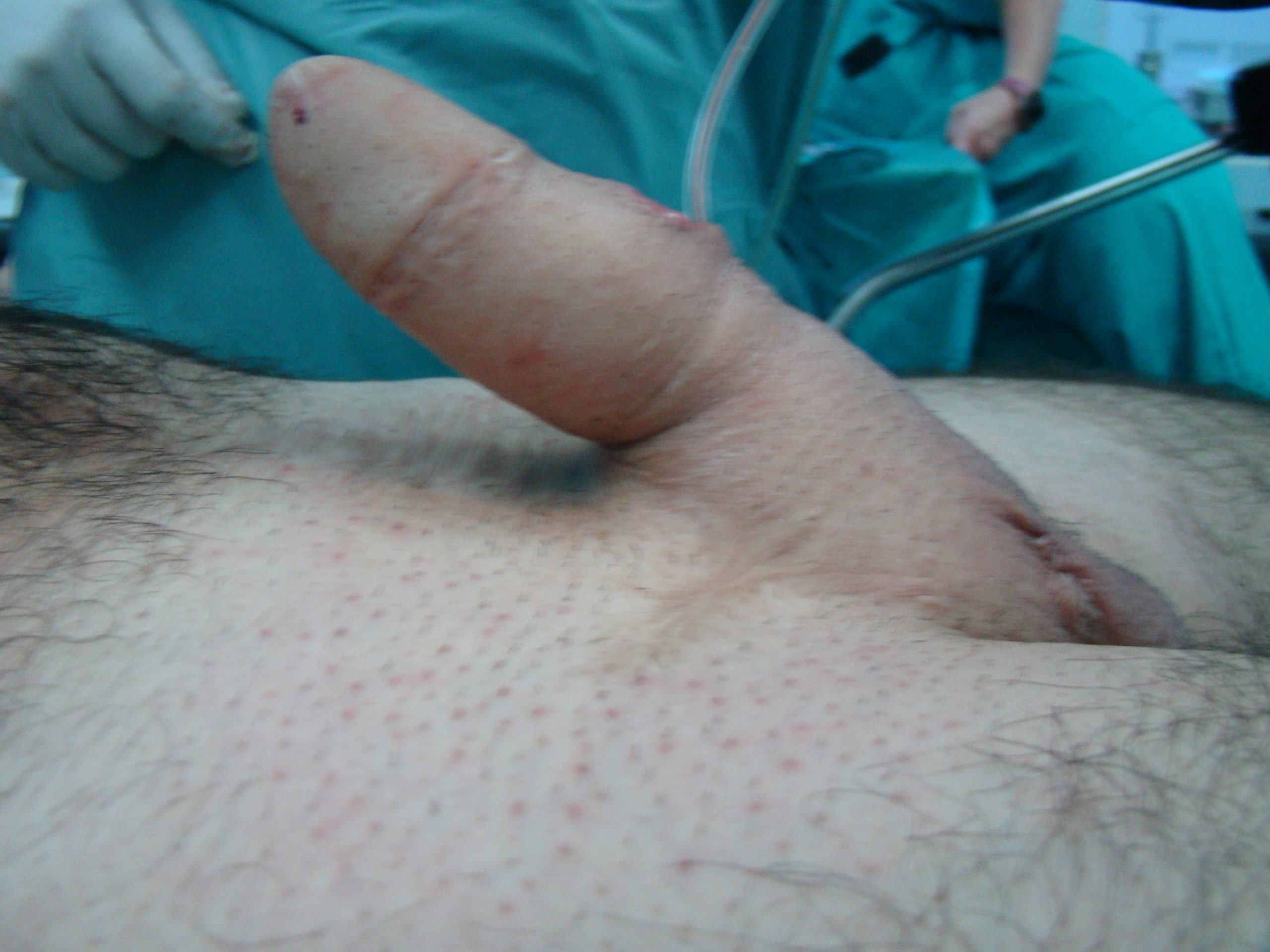
Result after implantation of semirigid two cylinder penile prosthesis.
1. Bencic M, Stojanovic B, Bizic M, Djordjevic ML. MUSCULOCUTANEOUS LATISSIMUS DORSI PHALLOPLASTY. Indian J Plast Surg. 2022. epub 24 June 2022
2. Purohit RS, Kent M, Djordjevic ML. PENILE PROSTHESIS IN TRANSGENDER MEN AFTER PHALLOPLASTY. Indian J Plast Surg. 2022. epub 24 June 2022
3. Djordjevic ML. REEGRETS IN TRANSGENDER FEMALE: REVERSAL PHALLOPLASTY. In: Nikolavsky, Blakely (Eds). UROLOGICAL CARE FOR THE TRANSGENDER PATIENT. 2021; pp 229-236. Cham, Switzerland: Springer International Publishing AG.
4. Stojanovic B, Bizic M, Djordjevic ML. URETHRAL RECONSTRUCTION IN FEMALE TO MALE GENDER AFFIRMING SURGERY. In: Martins F, Kulkarni S, Tobias R, (Eds). TEXTBOOK OF MALE GENITOURETHRAL RECONSTRUCTION. 2020; pp 883-896. Cham, Switzerland: Springer International Publishing AG.
5. Al-Tamimi M, Pigot GL, van der Sluis WB, van de Grift TC, van Moorselaar RJA, Mullender MG, Weigert R, Buncamper ME, Özer M, de Haseth KB, Djordjevic ML, Salgado CJ, Belanger M, Suominen S, Kolehmainen M, Santucci RA, Crane CN, Claes KEY, Monstrey S, Bouman MB. THE SURGICAL TECHNIQUES AND OUTCOMES OF SECONDARY PHALLOPLASTY AFTER METOIDIOPLASTY IN TRANSGENDER MEN: AN INTERNATIONAL, MULTI-CENTER CASE SERIES. J Sex Med. 2019;16(11):1849-1859.
6. Bizic M, Djordjevic ML. GENITAL GENDER CONFIRMATION SURGERY FOR PATIENTS ASSIGNED FEMALE AT BIRTH. In: Cecile AF (Ed), COMPREHENSIVE CARE OF THE TRANSGENDER PATIENT. 2019; pp 93-113. Amsterdam, Netherlands: Elsevier.
7. Djordjevic ML, Bencic M, Kojovic V, Stojanovic B, Bizic M, Kojic S, Krstic Z, Korac G. MUSCULOCUTANEOUS LATISSIMUS DORSI FLAP FOR PHALLOPLASTY IN FEMALE TO MALE GENDER AFFIRMATION SURGERY. World J Urol. 2019;37(4):631-637.
8. Djordjevic ML. NOVEL SURGICAL TECHNIQUES IN FEMALE TO MALE GENDER CONFIRMING SURGERY. Transl Androl Urol 2018;7(4):628-638.
9. Jun M, Pušica S, Kojovic V, Bizic M, Stojanovic B, Krstic Z, Korac G, Djordjevic ML. TOTAL PHALLOPLASTY WITH LATISSIMUS DORSI MUSCULOCUTANEOUS FLAP IN FEMALE TO MALE TRANSGENDER SURGERY. Urology. 2018;120:269-270.
10. Djordjevic ML, Stojanovic B. TOTAL PHALLOPLASTY WITH LATISSIMUS DORSI MUSCULOCUTANEOUS FLAP IN FEMALE-TO-MALE TRANSGENDER. In: Tran TA, Panthaki ZJ, Hoballah JJ, Thaller SR (Eds.), OPERATIVE DICTATIONS IN PLASTIC AND RECONSTRUCTIVE SURGERY. 2017; pp 577-583. Cham, Switzerland: Springer International Publishing AG.
11. Bizic MR, Stojanovic B, Djordjevic ML. Genital reconstruction for the transgendered individual. J Pediatr Urol 2017;13:446-52.
12. Djordjevic ML, Bizic MR, Duisin D, Bouman MB, Buncamper M. Reversal surgery in regretful male-to-female transsexuals after sex reassignment surgery. J Sex Med 2016; 13(6): 1000-7
13. Djordjevic ML. Primary and secondary reconstruction of the neophallus urethra. In: Brandes SB and Morey AF, Eds. Advanced male urethral and genital reconstructive surgery, 2nd Edition. New York: Humana Press; 2014. p. 493-505.
14. Djordjevic ML, Salgado CJ, Bizic M, Kuehhas FE. Gender dysphoria: the role of sex reassignment surgery. ScientificWorldJournal. 2014;2014:645109.
15. Djordjevic M, Kojic S, Stanojevic D, Jocic D, Bizic M. Total phalloplasty in female transsexuals: technique and outcomes. Eur Urol Suppl 2011;10(9):579.
16. Djordjevic ML, Bizic M, Stanojevic D, Bumbasirevic M, Kojovic V, Majstorovic M, Acimovic M, Pandey S, Perovic SV. Urethral lengthening in metoidioplasty (female-to-male sex reassignment surgery) by combined buccal mucosa graft and labia minora flap. Urology 2009; 74:349-353
17. Djordevic M, Stanojevic D, Kojovic V, Bizic M, Majstorovic M, Pandey S, Total phalloplasty combined with metoidioplasty as a single stage procedure in female to male gender reassignment surgery: preliminary report. XXI Biennial Symposium WPATH, Oslo, Norway, 2009; p40.
18. Kojovic V, Bizic M, Majstorovic M, Kojic S, Stanojevic D, Korac G, Djordjevic M. Combined total phalloplasty and metoidioplasty as a single stage procedure in female to male gender reassignment surgery. EurUrolSuppl, 2009;8(8):648